Multiple sclerosis (MS) is a complex neurological condition that involves both inflammatory demyelinating and neurodegenerative components. MS research and treatments have traditionally focused on immunomodulation, with less investigation of neuroprotection, and this holds true for the role of vitamin D in MS. Vitamin D plays an anti-inflammatory role in modulating the immune system in MS. More recently, researchers have begun investigating the potential neuroprotective role of vitamin D in MS, which may be important in remyelination and/or the prevention of demyelination. There is a growing body of research uncovering mechanistic role of vitamin D-mediated neuroprotection, including: enhancing oligodendrocyte lineage differentiation, enhancing neurotrophin expression, attenuating aberrant microglial and reactive astrocyte activation, stabilizing the BBB, and reducing oxidative stress.
Here, we focus on the most notable of these mechanisms, that 1,25(OH)2D3 promotes stem cell proliferation and drives the differentiation of neural stem cells into oligodendrocytes, which carry out remyelination.
Vitamin D Promotes Oligodendrocyte Proliferation and Differentiation
Oligodendrocyte dystrophy and apoptosis are significant pathological features in the demyelinating lesions of MS [
51]. Oligodendrocytes are myelin-producing glial cells that support neurons in the CNS. During periods of tissue injury, mature oligodendrocytes have the ability to remyelinate CNS neuronal axons to maintain saltatory conduction, which is a prerequisite for proper brain functioning [
52]. Remyelination by oligodendrocytes can be robust and restorative, especially in early MS, but declines during later stages of the disease [
52,
53]. The ability to regenerate the oligodendrocyte population depends on the availability of neural stem cells (NSCs) and oligodendrocyte progenitor cells (OPCs) [
52]. Oligodendrogenesis is the process by which NSCs commit to an oligodendrocyte lineage and differentiate into OPCs, which ultimately differentiate into oligodendrocytes [
54]. However, in MS, especially during the progressive stage, the regenerative capacity of NSCs and OPCs to give rise to oligodendrocytes is considerably diminished, contributing to neuronal degeneration and impaired axonal conduction [
6,
53,
55].
It has previously been shown that OPCs and oligodendrocytes express VDR [
34]. VDR-RXR heterodimerization is present in OPCs and is necessary for OPC differentiation [
37,
56]. The capacity of 1,25(OH)
2D
3 to promote OPC differentiation is diminished in the presence of a VDR antagonist in a dose-dependent manner [
37]. By blocking VDR, it becomes apparent that 1,25(OH)
2D
3 is exerting its proposed neuroprotective effect on oligodendrocyte lineage cells via VDR-RXR signalling [
37]. In 2015, it was demonstrated that VDR is constitutively expressed in NSCs [
57]. Increasing vitamin D in vitro upregulates VDR expression in NSCs in a dose-dependent manner [
57].
Increased 1,25(OH)
2D
3 exposure stimulates an increase in NSC proliferation and, importantly, increases the proportion of NSCs that can differentiate into oligodendrocyte lineage cells [
57,
58] (
Figure 1). In a study utilizing a lysolecithin-induced model in the corpus callosum of male rats, the group that received oral 1,25(OH)
2D
3 had a higher concentration of OPCs at lesion sites compared to sham and control groups [
58]. Furthermore, 1,25(OH)
2D
3 administration increases the proportion of mature oligodendrocytes in a cuprizone-induced demyelination model as well as in NSC and OPC cultures [
37,
57,
59]. Consistent results were found in a murine experimental autoimmune encephalomyelitis (EAE) model, where 1,25(OH)
2D
3 administration increased the concentration of NSCs, OPCs, and oligodendrocytes [
60]. EAE models are often considered more reflective of MS pathogenesis than demyelination models as they exhibit both immune-mediated inflammation and demyelination [
61]. Additionally, 1,25(OH)
2D
3 administration upregulates the expression of myelin basic protein and proteolipid protein, which are markers of myelin content [
37,
58,
59,
60]. The upregulation of these markers may suggest that demyelination is reduced and/or remyelination is increased in response to 1,25(OH)
2D
3 [
37,
58,
59,
60].
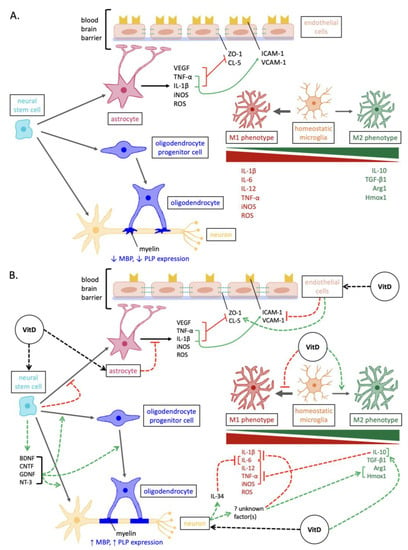
Figure 1. Overview of the mechanisms involved in neurodegeneration versus vitamin D-mediated neuroprotection in MS. (A) Pathways of neurodegenerative pathogenesis in MS. In addition to the depicted, the expression of neurotrophins and antioxidant enzymes is reduced in neurons and glia. (B) Neuroprotective pathways elicited by vitamin D in MS. Abbreviations: Arg1, arginase 1; BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; CL-5, claudin-5; GDNF, glial cell line-derived neurotrophic factor; Hmox1, heme oxygenase 1; ICAM-1, intercellular cell adhesion molecule-1; iNOS, inducible nitric oxide synthase; IL, interleukins; MBP, myelin basic protein; NT-3, neurotrophin-3; PLP, proteolipid protein; ROS, reactive oxygen species; VCAM-1, vascular cell adhesion molecule-1; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; ZO-1, zonula occluden-1.
Enhancing Neurotrophin Expression in Oligodendrocytes
Reduced neurotrophin secretion is another factor that contributes to inadequate neuroprotection in neurodegenerative disorders such as MS [
62,
63]. Neurotrophins are a family of proteins that elicit protective and regenerative effects by stimulating the proliferation and differentiation of NSCs, as well as the growth, survival, and proper functioning of neuronal and glial cells [
62,
64,
65]. Neurotrophins are secreted by multiple cell types, some of which include oligodendrocytes, among other cell types [
57,
65,
66,
67,
68]. Key neurotrophins include NT-3, BDNF, CNTF, GDNF, and NGF [
64].
Vitamin D has previously been demonstrated to increase neurotrophin expression [
69,
70,
71]. When mouse NSCs were cultured with 1,25(OH)
2D
3, the expression of NT-3, BDNF, CNTF, and GDNF was upregulated [
57]. Consistent results were observed in rodent models showing the upregulation of NGF and BDNF in CNS tissue following 25(OH)D
3 supplementation [
71,
72,
73]. In addition, 1,25(OH)
2D
3 exposure stimulated oligodendrogenesis and neurogenesis in mouse NSCs [
57]. This effect may be mediated by the induction of these neurotrophins, whereby 1,25(OH)
2D
3 enhances NSC proliferation and differentiation into neurons and oligodendrocytes [
57]. These neurotrophins have all been previously associated with enhanced oligodendrogenesis and neurogenesis [
74,
75,
76,
77,
78,
79,
80]. Overall, increased neurotrophin secretion in response to vitamin D may tip the balance towards a less neurotoxic environment in which CNS cells can more effectively contribute to repair and regeneration (
Figure 1).
Reducing Reactive Astrogliosis
Astrocytes comprise a large portion of the glial cell population in the CNS [
110]. They elicit essential functions, such as metabolically assisting neuronal growth, signalling immune cell entry into the CNS, and forming a critical component of the BBB [
110]. When CNS injury occurs, astrocytes become reactive and divide rapidly, also termed astrogliosis, which has both positive and negative consequences [
110,
111]. Reactive astrocytes aid in recovery by encompassing the site of demyelination, resulting in the construction of a glial scar, which prevents the injury from expanding [
112]. However, after a certain point, the abnormal increase in the number of reactive astrocytes is detrimental, as it contributes to the development of MS lesions [
110,
113,
114]. Reactive astrocytes release a number of pro-inflammatory cytokines and ROS, which can be neurotoxic neurons, OPCs, and oligodendrocytes [
115].
Reducing the activation and abundance of astrocytes may make the neurodegenerative microenvironment more conducive to repair processes, including the impact on oligodendrocytes [
67,
116]. MS plaques with fewer reactive astrocytes exhibit elevated OPC content and greater remyelination [
116]. The expression of VDR and CYP27B1 were upregulated in the astrocytes of LPS-stimulated rats, supporting a potential response via vitamin D [
36]. In rodent models of cuprizone-induced demyelination and LPS injection, it was shown that the concentration and activation of astrocytes were decreased in mice that were administered intraperitoneal injections of 25(OH)D
3 and 1,25(OH)
2D
3 [
36,
59]. Findings from other rodent CNS disease models have similarly supported a decrease in GFAP expression and astrocyte activation upon supplementation with oral or injected vitamin D [
36,
71]. More specifically, 25(OH)D
3 and 1,25(OH)
2D
3 downregulated iNOS, TLR4, TNF-α, and IL-1β in cultured astrocytes and EAE [
36,
117,
118] (
Figure 1). Additionally, the in vitro exposure of mouse NSCs to 1,25(OH)
2D
3 reduces NSC differentiation into astrocytes [
57] (
Figure 1). This is interesting, as vitamin D has the opposite effect of increasing NSC differentiation into oligodendrocytes and neurons (discussed above) [
57], consistent with its role in neuroprotection.
Stabilizing the Blood–Brain Barrier: impact on oligodendrocytes
The BBB regulates the movement of blood-borne molecules, ions, and cells into the CNS, leading to the stabilization and protection of the neuronal microenvironment [
119,
120,
121,
122]. Breakdown of the BBB and consequent hyperpermeability occurs early in MS [
123]. When stimulated by pro-inflammatory cytokines from various immune cells, endothelial cells of the BBB downregulate tight junctions and upregulate cell-adhesion molecules, which destabilizes the BBB and increases leukocyte recruitment into the CNS, respectively [
124]. Reactive astrocytes also play a role in BBB instability [
120,
125,
126]. Pro-inflammatory cytokines, including TNF-α and IL-1β, secreted from reactive astrocytes stimulate the endothelial cells to downregulate tight junctions and upregulate cell-adhesion molecules [
120,
125,
126]. The reactive astrocytes also detach their endfeet processes from the capillary endothelium, making the BBB more permeable [
127]. Interestingly, in a neurodegenerative environment, reactive astrocytes release vascular endothelial growth factor (VEGF), which signals endothelial cells to lower tight junction expression, which destabilizes the BBB [
128]. As a result of BBB hyperpermeability, CD4+ Th1 and Th17 cells are able to translocate into the CNS, where their secreted cytokines prompt the degeneration of oligodendrocytes and myelinated axons [
123].
Vitamin D is thought to counteract BBB hyperpermeability through multiple mechanisms (
Figure 1). In a study using human brain endothelial cells, the effects of 1,25(OH)
2D
3 exposure were examined following exposure to TNF-α and exposure to sera derived from MS patients [
129]. It was found that 1,25(OH)
2D
3 can act directly on endothelial cells to upregulate tight junction proteins (zonula occluden-1 and claudin-5) and downregulate cell adhesion molecules (ICAM-1 and VCAM-1) [
129]. These two outcomes both contribute to BBB stabilization [
129].
In addition to reducing BBB permeability by upregulating tight junction proteins and downregulating cell-adhesion molecules, 1,25(OH)
2D
3 has been shown to lower the expression of matrix metalloproteinase-9 (MMP-9) in mouse-brain endothelial cells and in a rat model of ischemic stroke [
130,
131]. Various cell types, including endothelial cells, CNS cells, and leukocytes, release MMPs [
132]. MMPs are responsible for breaking down extracellular matrix components (such as collagen, fibronectin, and laminin) and tight junction proteins, thereby contributing to BBB instability [
132,
133,
134,
135]. As such, reducing MMP-9 expression may be another underlying mechanism by which vitamin D promotes BBB stabilization [
98]. It has also been demonstrated that 1,25(OH)
2D
3 reduces the apoptosis of human endothelial cells exposed to MS sera, which may indicate a further protective effect of vitamin D on the BBB [
98,
136].
In relation to oligodendrocyte-related neuroprotection, Stabilization of the blood brain barrier subsequently helps prevent the degeneration of oligodendrocytes.
Overall, the neuroprotective effects elicited by vitamin D promote a more stable microenvironment in which CNS glial cells can more easily participate in repair and recovery processes. In particular, 1,25(OH)2D3 promotes stem cell proliferation and drives the differentiation of neural stem cells into oligodendrocytes, which carry out remyelination. This could potentially support and restore functioning of neurons in MS.
This entry is adapted from the peer-reviewed paper 10.3390/nu15132978