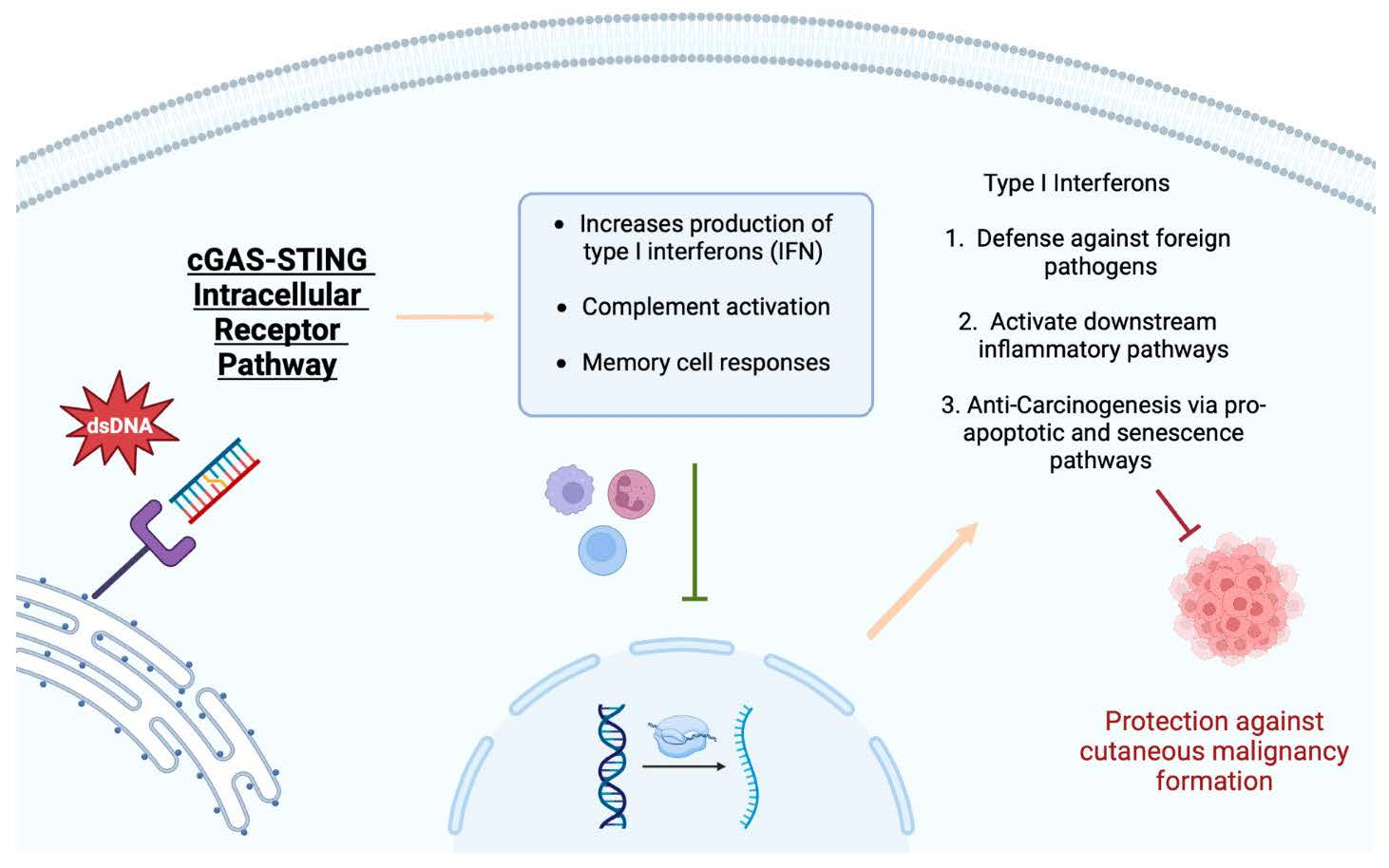
The cGAS-STING signaling pathway has gained significant attention regarding its anti-tumor properties in certain malignancies, especially skin cancer. STING plays a vital role in the fight against foreign pathogens, the activation of downstream inflammatory pathways, and anti-carcinogenesis via pro-apoptotic and senescence pathways. The STING pathway functions by detecting the presence of DNA, which subsequently leads to the activation of inflammatory modulators for host defense, anti-inflammatory, and anti-oncogenic effects.
1. Stimulator of Interferon-Related Genes
New technological capabilities have brought the STING signaling pathway to the forefront of the oncology world. The pathway was first described in 2008 as a eukaryotic defense mechanism against viruses [
20]. Since this time, the pathway has gained notoriety for its activity against different types of cancer and its ability to potentiate oncologic therapies. At a fundamental level, the pathway is an inflammatory response to double-stranded DNA [
20]. Specifically, STING upregulates type 1 IFN production [
7,
20].
The human immune system’s normal response to foreign antigens is accomplished by two distinct parts of the immune system. Physical barriers, including skin and mucosal membranes, comprise the innate immune system, providing physical and molecular protection defenses against invading organisms and molecules. Conversely, the adaptive immune system comprises cells with specialized functions that interact in order to produce antibodies, activate complement, and activate memory responses in order to identify and eliminate pathogens. Antigens from foreign molecules are ingested and processed by antigen-processing cells, which present these molecules to specialized T-cells via major histocompatibility complexes. Once presented, the antigens can then go on to activate other cells, molecular cascades, signaling pathways, and cytotoxic host responses.
The immune system produces various inflammatory cytokines and IFNS in response to acute pathogenic invasion. Of note, there are currently three major IFN classes. Although each family exhibits differences in potency, receptor type, and specific downstream messengers, the STING pathway is most closely intertwined with type 1 IFNs.
Type 1 IFNs allow host cells to defend themselves against foreign viruses, bacteria, and fungi and can activate other downstream inflammatory pathways [
21]. This class has also been hypothesized to exhibit anti-malignancy properties; recent studies have focused on identifying a stimulus to promote a Type 1 IFN response. An intracellular receptor resides within the endoplasmic reticulum and activates type 1 IFNs via a well-documented cGAS-STING pathway [
22]. Importantly, type 1 IFNs have also been implicated in the pathogenesis of other diseases, including pulmonary disease [
21].
If non-native double-stranded DNA is detected within the cytoplasm of a host cell, the cGAS-STING pathway is initiated [
23]. Once the foreign material encounters cGAS, a sensing protein, a conformational change occurs, facilitating the formation of a molecule of 2′,3′-cyclic GMP-AMP. Additionally, this step leads to an interaction between GTP and ATP, which ultimately results in the activation of STING, which is housed within the endoplasmic reticulum when inactive [
23,
24]. Simultaneously, palmitoylation of TANK-binding kinase 1 occurs, which leads to the recruitment of IFN regulatory factor 3 and the phosphorylation of STING [
23,
24]. This sequence is hypothesized to cause a conformational change, allowing STING to translate through the Golgi apparatus and move towards the perinuclear region via the assistance of several modulators, including iRhom2 [
23,
24]. The final event in this sequence is the translocation of IFN regulatory factor 3, which travels to the nucleus. Once it reaches its destination, the transcription of Type 1 IFN genes is stimulated.
Figure 1 summarizes the modulation of skin cancer by the stimulator of interferon genes.
Figure 1. The modulation of skin cancer by the stimulator of interferon genes.
2. Role of STING in DNA Damage
The previously mentioned innate immune system reacts to foreign nucleic acids via RNA and DNA sensing receptors to mount a host immune response [25]. DNA infiltration into cells can propagate various changes, including senescence, replication, mitochondrial stress, and others that lead to the up-regulation of type I IFN [25]. Activation of pattern recognition receptors (PRRs), specifically the cGAS/STING pathway, is activated when the presence of double-stranded DNA (dsDNA) of foreign invaders is detected [25]. The identification of dsDNA as a signaling mechanism for STING activation has prompted interest in its ability to be a therapeutic target against carcinogenesis. Downstream of the STING signaling pathway lies its effects on host cell DNA [25].
Mitochondrial DNA damage also activates this pathway, and degradation has been shown in malignancies to trigger cGAS-STING. Cytosolic mitochondrial DNA release acts as a ligand for pathway potentiation, causing IFN-mediated cell death that may be a target for future therapies [
25,
27]. This immunologic role of cGAS and STING has been demonstrated to be diminished in lung adenocarcinoma, late-stage gastric cancer, and invasive breast ductal carcinoma, leading to poor survival [
25,
28,
29,
30].
Cancers evade the cGAS-STING-mediated signaling once DNA is sensed via targeting gene expression or suppressing their function. Suppression of cGAS-STING signaling downregulates apoptotic and senescence pathways, increasing the protection of malignant cells from host tumor surveillance [
25,
31]. Under hypoxic conditions, cGAS-STING signaling decreases simultaneously with the release of mitochondrial DNA, both leading to muted anti-tumor responses, further evidencing the necessary role of STING signaling for anti-tumorigenesis [
25,
28,
32]. Increased host vulnerability in these states also leaves host cells more susceptible to oncogenic viral replication, such as in the herpes simplex virus.
3. Role of STING in Immunotherapy of Skin Cancer
Given the exhibited anti-oncogenic properties of STING, it is no surprise that new advances in oncology have investigated this pathway to target skin cancer tumorigenesis. Several studies have explored how STING responds to damaged DNA released from lysed tumor cells [
33]. In addition to decreased DNA repair mechanisms, tumor cells exhibit replicative immortality, incorporating the nucleoside analog 6-thio-dG into the growing telomeres [
34]. This nucleoside can become imbalanced, also resulting in damaged DNA that can be detected by the normal cells in the body [
34]. This leads to the activation of IFNa and IFNb, which ultimately increases the presentation of oncogenic antigens to the recruited CD8+ T-cells while promoting additional cell apoptosis through NK cells [
33]. By understanding that the STING pathway is driven by damaged dsDNA, therapies for skin cancer may be able to target the accessibility and recognition of the dsDNA by antigen-presenting cells.
3.1. Melanoma
Although checkpoint inhibitor therapies currently exist to target skin cancer, their primary mechanisms do not primarily rely on inducing damage to dsDNA in tumor cells. Therapies for malignant skin cancer, such as radiotherapy and chemotherapy, rely on the STING pathway for regulating type 1 IFN production. Other documented mechanisms such as inducing DNA damage and forming micronuclei, inducing apoptosis, and exposing the damaged DNA to PPRs on the cell surface have been described [
35]. Tumor cells have been found to suppress STING’s activity, resulting in resistance to these treatments [
36,
37].
Drugs that target, the DNA repair enzymes or replication interfere with producing dsDNA recognizable to the host cell, thus activating the STING pathway [
38,
39]. Topoisomerase inhibitors have been found to promote antigen expression in multiple melanoma lineages and T-cell induction of IFNs due to their ability to promote abnormal DNA replication [
40]. Damaged dsDNAs, produced by drugs that impede DNA repairs and replication, ultimately stimulate the STING pathway and, therefore, can be seen as potential therapies in the induction of the STING pathway in the fight against skin cancer.
STING agonists are another type of immunotherapy currently being studied for their efficacy on the tumor microenvironment of the integumentary system. In one study, when STING agonists were injected intratumorally into subcutaneous melanomas, the pathway increased the production of local anti-angiogenic factors, chemokines, and LTbR agonists, ultimately aiding in the restoration of the normal vasculature and promotion of local tertiary lymphoid structures resulting in the slow growth of the tumor microenvironment [
41]. Although the external validity of this study was limited to murine species, human STING agonists that target skin tumors are currently being developed, and have exhibited positive results in clinical trials [
42]. However, further development of these agonists is needed in order to increase their responsiveness and manipulate the mechanism of agonist entry into the cells due to underlying hydrophilicity [
33].
3.2. Squamous Cell Carcinoma
There are limited non-surgical options for treating SCCs, with radiation therapy being a popular treatment for this cancer [
46]. Similar to melanoma, CTLA-4/PD-1 inhibitors are the primary immunotherapy treatment. STING has been found to stimulate the PD-1 pathway. Therefore, STING therapy with CTLA-4/PD-1 inhibitors may further increase the efficacy of prohibiting cancerous growth [
7]. Low expression of STING has also been found to be associated with worse outcomes in squamous cell carcinomas located on the head and neck regions [
47].
3.3. Basal Cell Carcinoma
Like SCC, radiation and topical chemotherapy are amongst the more popular non-surgical options for BCC. There are currently no FDA immunotherapy approvals for advanced or metastatic BCCs [
48]. Due to the main etiological factor for BCC being UV exposure, this NMSC is a prime target for the STING pathway due to the dsDNA damage precipitated by sun exposure. Research has found that IFNa treatment induces BCC regression by inducing suicide through CD95 receptor–CD95 ligand interaction [
49]. These findings emphasize the need for future therapy development manipulating the STING pathway as STING pathogenesis includes activation of IFNs such as IFN-a.
3.4. STING Resistance in Tumor Cells
STING is vital in prohibiting tumor growth, but to adapt to this, tumor cells have evolved to inhibit aspects of the STING pathway. Research has found that CD8+ T-cells in cancer patients have decreased expression of the STING pathway and are less successful in promoting an anti-oncogenic response. Therefore, when the pathway in CD8+ T-cells that have decreased expression of the pathway was stimulated, the cells were more successful in promoting an anti-tumor response by increasing differentiation of stem-cell CD8+ cells [
34]. Tumor cells have also targeted epigenetic modifications and degradation of the STING pathway to suppress its anti-oncogenic effects [
50,
51]. Research has found that loss of STING function prohibited melanoma cells from producing type 1 IFNs after exposure to damaged dsDNA [
49].
4. Further Potential Therapies
Further potential therapies include the injection of mRNA-lipid nanoparticles of constitutively active STING mutants into the cancer cells, which have been found to reactivate STING anti-tumor immunity and promote apoptosis of tumor cells [
52]. This mechanism of action does not induce anti-proliferative effects in lymphocytic cells that could result in cytotoxicity as seen with STING agonists and proves to be a potential therapy to treat “cold tumors”. Potential therapies that have yet to be explored include targeting cytosolic DNA sensors such as AIM2 that have an antagonistic effect on the STING pathway [
53]. Further research with STING agonist combination therapy includes combining the agonist with a protein-based cancer vaccine [
54]. Positive results were observed as this promising therapy caused IFN and TNFa production levels, promoting CD8+ and CD4+ T-cell infiltration and function while polarizing CD4 T-cells towards TH1 differentiation [
54]. Additionally, STING combined with a cancer vaccine was shown to decrease the presence of immune-suppressive cells surrounding the tumor; however, most of the oncogenic cells escaped from immune surveillance [
54]. Therefore, further modification of this therapy is required in order to increase its efficacy and minimize tumor escape. The role of cGAS-STING activation, when paired with existing cancer treatments, poses beneficial potential in maximizing responses. The synergistic effects of these vaccines, if combined with chemotherapy or radiotherapy, and immune checkpoint blockade (ICB) therapy are of particular interest. Recent work has suggested that radiation therapy enhances anti-tumorigenesis via immune activation by cGAS-STING. Resistance mechanisms against this have been demonstrated [
25,
55,
56,
57,
58]. However, it has been proposed that combinations of radiotherapy with cyclic dinucleotides (CDNs) like c-di-GMP and cGAMP acting as downstream second messengers for STING activation might enhance tumor clearance [
25]. Where it has been shown that CDN monotherapy can suppress tumor growth via innate immune signaling, combining with radiotherapy allows for CD8+ T-cell involvement and synergy [
25,
59]. Vaccine combinations with irradiated tumor cells that display GM-CSF and CDNs have also exhibited anti-oncogenic responses in melanoma, SCC, and other non-cutaneous malignancies [
25,
60]. Regarding immune checkpoint inhibitors, it has been observed that STING-deficient mice respond poorly in comparison to wild-type mice undergoing anti-CTLA4 and anti-PDL1 immunotherapies [
25,
61,
62,
63,
64]. Thus, CDN-induced STING signaling combined with ICB therapies also enhanced the CD8+ T-cell response and anti-tumor attenuation [
65].
This entry is adapted from the peer-reviewed paper 10.3390/genes14091794