1. Introduction
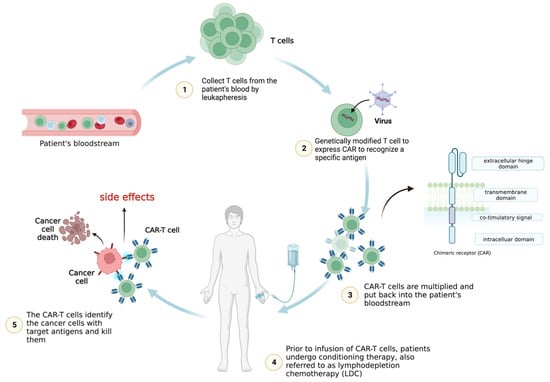
Leukapheresis is a procedure used to extract T cells from the patient’s blood in the initial treatment stages. After being collected and separated, the T cells are engineered in vitro to generate a CAR to detect specific antigens. The CAR-T cells are then multiplied in a laboratory to increase their number. Prior to the CAR-T cell reinfusion, patients may undergo conditioning therapy, also referred to as lymphodepletion chemotherapy (LDC) [
2,
3].
Radiotherapy or chemotherapy may be required to eliminate the patient’s immune cells, especially T cells that could compete against CAR-T cells for cytokines, nutrition, or other resources. The patient’s blood undergoes infusion once more with engineered CAR-T cells, which can travel to the malignant location and attack the tumor cells that express antigens recognized by the CAR. After the infusion, the effectiveness of CAR-T cell therapy and its side effects are closely monitored in the patients (Figure 1).
Figure 1. CAR-T cell therapy. The CAR-T cells are expanded before infusion into the patient’s bloodstream following lymphodepletion chemotherapy (LDC). Once in the body, the genetically modified chimeric antigen receptors (CARs) allow the T cells collected from the patient to recognize and attack cancer cells expressing specific antigens.
CAR-T cell therapy has demonstrated exceptional therapeutic effectiveness in patients with leukemia and B cell lymphoma who have relapsed or been resistant to treatment, showing high remission rates and exhibiting tremendous potential for treating additional hematological malignancies [
4,
5,
6]. Despite these encouraging findings, therapy-related severe adverse effects are frequent and can be fatal [
7].
2. Cytokine Release Syndrome (CRS)
The most severe prevalent toxicity in CAR-T cell therapy is an overall inflammatory reaction termed cytokine release syndrome (CRS) [
8,
9,
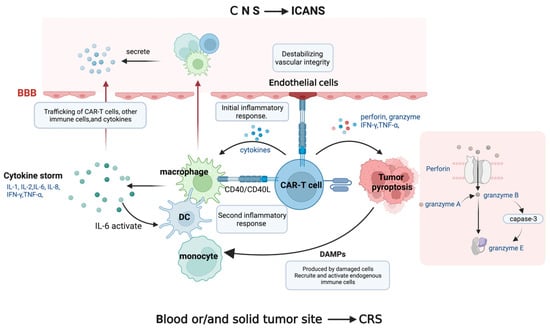
10]. Tumor antigen recognition triggers CAR-T cell activation and CRS, a state of severe systemic inflammation. Following CAR-T cell injection, patients’ serum levels of cytokines, including interferon-gamma (IFN-γ), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-2, IL-8, and IL-10, are produced by CAR-T cells and elevated, resulting in a cytokine storm. This is followed by a second inflammatory response involving antigen-presenting cells (APCs) such as dendritic cells (DCs), B cells, macrophages, and monocytes displaying the cell surface protein CD40. On activated CAR-T cells, the ligand for CD40 (CD40L) is also substantially expressed.
APC recognition favors the binding of CD40 and CD40L, which is contact-dependent, improves APCs’ antigen presentation, and stimulates the release of cytokines, including IL-1β, IL-6, and TNF-α [
11,
12]. Among these cytokines, IL-6 contributes to cytokine synthesis, stimulating DCs, macrophages, monocytes, and other bystander immune cells, which release additional cytokines. This phenomenon results in an enhanced inflammatory response. It creates a positive feedback loop that supports cytokine release, which may have important consequences for the onset and development of inflammatory illnesses. Robust IL-6 secretion leads to more severe CRS symptoms. The greater the IL-6 secretion, the more severe the CRS. Indeed, IL-6 is presently considered the primary contributor to toxicity in CRS [
13].
Activated endothelial cells release IL-6 and endothelial permeability factors in the setting of hyperinflammation [
14]. This also mediates hemodynamic dysfunction, a capillary leak, and consumptive coagulation disorders in CRS patients by destabilizing vascular integrity. Furthermore, it should be noted that endothelial cells can be activated by CD40, thereby implicating CD40/CD40L interactions that result in the upregulation of adhesion molecules and increased angiopoietin-2, resulting in organ malfunction, capillary leakage, tissue edema, and hypotension (
Figure 2) [
15,
16].
Figure 2. The underlying mechanisms of CRS and ICANS. CAR-T cells produce cytokine storm, which activates bystander immune cells. When CD40L on CAR-T cells interacts with CD40 on immune cells and endothelial cells, an inflammatory response occurs, with interleukin-6 (IL-6) playing a major role in the cytokine release positive feedback loop, compromising vascular integrity. The perforin produced by CAR-T cells induces tumor pyroptosis, releasing danger-associated molecular patterns (DAMPs). CAR-T cells, immune cells, and cytokines can penetrate the disrupted blood–brain barrier (BBB) and trigger inflammatory reactions in the central nervous system (CNS), resulting in neuronal injury.
In addition to the inflammatory response caused by immune cells, tumor cell pyroptosis during CAR-T cell therapy also causes CRS. As previously mentioned, CAR-T cells secrete perforin, granzyme, TNF-α, and IFN-γ following antigen recognition. Granzyme A and B enter the cytoplasm through the pores formed by perforin molecules. CAR T cells quickly activate caspase 3 in target cells by releasing granzyme B, which cleaves gasdermin E and causes extensive pyroptosis [
17]. In the meantime, gasdermin B is activated and cleaved by granzyme A from cytotoxic lymphocytes, causing target cell pyroptosis [
17,
18]. As a result, components released during pyroptosis induce macrophages to promote caspase 1 for gasdermin D cleavage, which causes the amplification of cytokines and eventual CRS. After cancer cell pyroptosis, these damaged and dying cells create or release danger-associated molecular patterns (DAMPs). The endogenous immune cells, including macrophages and dendritic cells, are subsequently recruited, and activated by these DAMPs, amplifying the inflammatory response, and increasing the release of cytokines like IL-1β and IL-6. This series of events sheds light on the intricate mechanisms behind CAR-T cell therapy’s therapeutic effect [
19].
The clinical manifestation of CRS occurs when a substantial number of myeloid cells or immunological cells become activated and release inflammatory cytokines [
20]. The triggering factor and the activation level of immune cells determine when symptoms will start to appear and how severe CRS will be. The initial symptom is almost invariably a fever, which can occasionally reach 40.58 °C or higher; some general signs are tiredness, malaise, and myalgias. After receiving CAR-T cells, the onset of fever can occur within a few hours to more than a week [
21]. Patients often show the first CRS symptoms 14 days after receiving CAR-T cells; delayed CRS is rarer [
22,
23]. After the onset of fever, patients may develop various hematologic and organ toxicities, some of which may be life-threatening [
24,
25,
26,
27], with capillary leaks causing pulmonary and peripheral edema, multiorgan failure, hypotension, and circulatory system collapse.
Hematologic toxicities are also prevalent following CAR-T cell injection. Disseminated intravascular coagulation, prolonged prothrombin time (PT), partial thromboplastin time (PTT), and decreased fibrinogen have all been reported. Hemorrhagic events after treatment with CAR-T cells have resulted in patient deaths [
26,
28,
29]. Moreover, following LDC, cytopenia is common, and delayed cytopenia is common after severe CRS [
30]. Hemophagocytic lymph histiocytosis (HLH) has been observed during CRS [
27], characterized with severe systemic hyperinflammation. Unremitting high fevers, cytopenia, coagulopathy, and an increase in typical HLH biomarkers are some of the defining characteristics of HLH.
Patients afflicted with CRS may experience wide-ranging organ dysfunction over time. Reports have indicated that myalgia is associated with increased creatine phosphokinase levels, which may suggest inflammatory muscle damage [
25,
31,
32]. Furthermore, temporary increases in bilirubin and liver enzymes have been noticed [
33,
34], indicating hepatitis and liver failure [
35]. Renal insufficiency may occur with a temporary rise in serum creatinine [
15]. Hypotension, arrhythmias, and left ventricular systolic dysfunction are the most frequent cardiac problems that occur after CAR-T cell therapies in both adult and juvenile populations, and these conditions can result in overt heart failure. CRS may worsen such occurrences, leading to undesirable cardiovascular outcomes [
36,
37].
The administration of CAR-T cells with the B cell maturation antigen (BCMA) target was associated with neutropenia, thrombocytopenia, and anemia. The patients also developed a grade 1 gastrointestinal hemorrhage and grade 1~2 hypoproteinemia, and hematological damage [
38]. Severe CRS cases can lead to a condition known as capillary leak syndrome (CLS). CLS involves fluid leakage from the bloodstream into surrounding tissues due to increased capillary permeability. This fluid shift can dilute the concentration of proteins in the blood, including albumin and other serum proteins, leading to hypoproteinemia, hypocalcemia, etc.
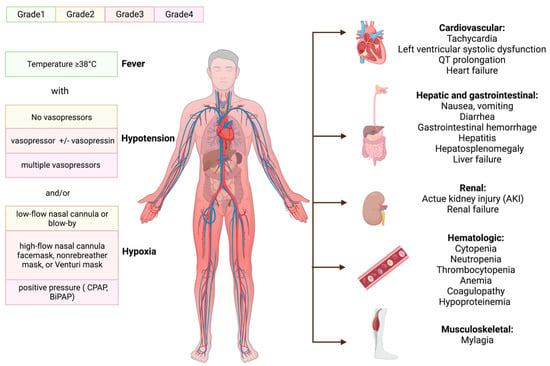
The American Society for Transplantation and Cellular Therapy (ASTCT) summarized the consensus guidelines for grading CRS, with the severity of CRS depending on the level of temperature, hypotension, and hypoxia (
Figure 3) [
39].
Figure 3. The manifestations of CRS and ASTCT CRS consensus grading. The initial fever may progress to numerous hematologic and organ toxicities before the clinical signs of cytokine release syndrome (CRS) appear. ASTCT classified it into four grades based on its chief manifestation: hypoxia, hypotension, and fever. Continuous positive pressure airway (CPAP); bilevel positive airway pressure (BiPAP).
3. Immune-Effector-Cell-Associated Neurotoxicity Syndrome (ICANS)
ICANS, formerly known as CAR-T-cell-related encephalopathy syndrome, is the second most frequent adverse impact of CAR-T cells. ICANS tends to be cytokine-mediated neurotoxicity rather than the OTOT impact, albeit the pathophysiology is still unknown. Several laboratory studies have proposed that endothelial activation contributes to ICANS pathogenesis [
40]. CRS-associated endothelial activation can disrupt the blood–brain barrier (BBB) and infiltration of the cerebrospinal fluid with inflammatory cytokines and leukocytes. The central nervous system (CNS) can become inflamed when cytokines and immune cells reach the brain parenchyma, impairing or damaging neurons. It is anticipated that CAR-T cells, monocytes, and macrophages will be drawn to the CNS and release cytokines, which constitute vital elements of ICANS [
41,
42,
43]. Mural cells, referred to as pericytes on the capillaries, are essential for maintaining the blood–brain barrier’s structure and encircle the endothelium, expressing CD19, which increases the BBB’s leakiness and causes ICANS in CD19-directed treatments [
44].
ICANS can be associated with various neurologic symptoms, yet these frequently evolve in a particular way. ICANS has been observed as early as the fourth or fifth day following CAR-T cell reinfusion and as late as the third or fourth week [
45,
46]. ICANS rarely develops in patients without prior CRS, but in these circumstances, it is often mild [
23]. ICANS encompasses headache, tremors, impaired speech, confusion, delirium, poor consciousness (obtundation, lethargy, and stupor), and, less frequently, localized abnormalities [
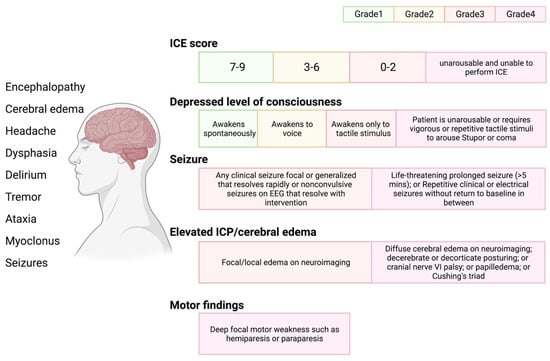
47]. The severity of ICANS is characterized and evaluated based on the American Society for Transplantation and Cellular Therapy (ASTCT) consensus criteria, which employ a method for assessing immunological functional cell-associated encephalopathy (
Figure 4) [
39].
Figure 4. ICANS and ASTCT CRS consensus grading for adults. Immune effector cell associated neurotoxicity syndrome (ICANS) includes multiple distinct neurologic symptoms. ASTCT graded ICANS into four categories based on an immune effector cell associated encephalopathy (ICE) score: depressed level of consciousness, seizure, elevated intracranial pressure (ICP)/cerebral edema, and motor findings.
4. Tumor Lysis Syndrome (TLS)
When a person experiences an effective cancer treatment (due to cell death), large amounts of phosphate, potassium, and nucleic acids can be released into their bloodstream, which can cause tumor lysis syndrome (TLS) [
48]. While some cases of TLS have been attributed to chemotherapy, CAR-T cell infusion has also led to acute anaphylaxis and TLS in some cases, even without prior conditioning chemotherapy [
27,
49,
50]. Indeed, rapid lymphoma cell death after CAR T cell treatment can be problematic if the kidneys cannot metabolize the byproducts of tumor cell lysis rapidly enough, resulting in hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia. Acute kidney damage can also be exacerbated due to the accumulation of uric acid, phosphates of calcium, and ferritin, resulting in systemic inflammation and iron overload.
5. On-Target, Off-Tumor Toxicity (OTOT)
Ideally, only the malignant cells will express a CAR-T cell target antigen, leaving healthy tissue unaffected. Solid tumors have had only patchy success with CAR-T cell treatment. A great deal of malignant antigens are tumor-associated antigens (TAAs), which are expressed on both healthy and tumor tissues. As a result, CAR-T cells are frequently unable to distinguish normal cells from malignant cells, resulting in the assault and death of normal cells, known as “on-target, off-tumor” toxicity [
51,
52]. OTOT is more likely to occur in solid tumors; therefore, greater research efforts are required to identify tumor-specific antigens (TSAs). In fact, several cases highlight the difficulties associated with TAA expression on normal tissue. For example, the first patient to receive CAR-T cells targeting HER2 experienced respiratory distress and a sizeable pulmonary infiltrate within 15 min of cell infusion, ultimately leading to lung damage and death [
10]. Lung toxicity was also observed in other clinical trials testing CAR-T cells directed against CEA [
51].
6. Additional Factors Associated with Toxicity
It is crucial to note that the incidence of side effects in CAR-T cell therapy might vary greatly based on the specific CAR-T cell product, the kind of cancer, and individual patient variables. In vivo, CAR-T cell expansion and toxicity may be exacerbated with tumor load, degree of conditioning therapy, higher infusion dose, and CAR design. Indeed, juvenile B-ALL patients with a higher baseline tumor burden exhibit more considerable CAR-T cell proliferation and severer CRS [
53,
54]. Large tumor loads in patients are also associated with higher severity and incidence of the syndrome, likely due to the higher levels of T cell activation observed in clinical studies [
34,
55]. Patients with a greater ALL burden and those who underwent higher infusion dosages of CD19 CAR-T cells were shown to have a higher incidence of CRS [
25,
56,
57].
CRS has been observed to begin earlier when CAR-T cells are constructed with CD28 costimulatory domains (as opposed to a 4-1BB costimulatory domain) [
33]. The CAR hinge region binds the scFv to the transmembrane part of the protein, and deleting the flexible amino acid glycine in this area may reduce CAR-T cell over-activation [
58]. The reduced flexibility in the CD8α hinge increased survival in preclinical investigations for high tumor burden and decreased proinflammatory cytokines, indicating potential benefits for safety and efficacy [
59,
60].
Enhancing the efficacy and duration of CAR-T cell responsiveness is the primary objective of conditioning therapy to improve the overall clinical outcomes in cancer patients. Although these negative consequences have also been recorded in the absence of chemotherapy conditioning, it is known that conditioned therapy is associated with the development of thrombocytopenia, anemia, and neutropenia [
31,
61,
62].
This entry is adapted from the peer-reviewed paper 10.3390/jcm12196124