You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Breast cancer is a complex disease for which pharmacological treatment does not guarantee success or cure. Plants possess phytochemical components with antioxidant and anti-inflammatory properties, mainly coumarins, flavonoids, alkaloids, and terpenoids. Several studies have demonstrated that bioactive compounds from plants can exert anti-tumor activity through various signaling pathways, such as apoptosis, autophagy, modification of the tumor microenvironment, cell arrest, and the suppression of angiogenesis.
- breast cancer
- phytochemicals
- herbal treatments
1. Introduction
Breast cancer is the most common malignancy worldwide and is the leading cause of death in women. In 2020, an estimated 19.3 million new cases of cancer were diagnosed, of which 11.7% were classified as breast cancer. Unfortunately, the mortality rate for this type of cancer was 6.9%. Although breast cancer mortality has decreased over the past 50 years due to improvements in diagnosis and treatment, it remains a global public health problem, particularly in developed countries [1]. The etiology of the disease is multifactorial and includes biological sex, age, level of economic development of the country of residence, hormonal status, genetic factors such as breast cancer gene dysfunction (BRCA1/2), consumption of processed foods, and obesity, among others [2,3].
The pharmacological treatment of breast cancer tumors depends largely on the type of tumor diagnosed [4,5,6]. However, their classification is far from simple. The classification of breast cancer tumors requires the following: (1) histopathologic analysis to determine the malignancy or degree of infiltration, the tumor architecture, and cytologic characteristics of the tumor, and (2) molecular analysis of characteristic markers, such as estrogen receptors (ERs), progesterone receptors (PRs), human epidermal growth factor receptor 2 (HER2), and (3) cell proliferation markers such as Ki67. These data are essential for determining tumor behavior and response to a given drug therapy [2,7,8,9].
Once the type of tumor has been determined, treatment usually involves a combination of different procedures, depending on the extent and severity of each patient’s breast cancer. There are local treatments (surgery and radiation therapy) and systemic treatments (chemotherapy, hormone therapy, and immunotherapy) [4,5]. Systemic treatments, such as chemotherapy, are designed to reduce the growth of highly proliferating cells that have spread throughout the patient’s body. The chemotherapeutic agents used for breast cancer can be divided into three antineoplastic classes: (1) those that act on DNA (alkylating agents such as cyclophosphamide; cytotoxic antibiotics such as doxorubicin; and antimetabolites such as 5-fluorouracil and camptothecin derivatives), (2) those that do not act on DNA but on extracellular cell division factors such as paclitaxel (taxane), and (3) bisphosphonates in the adjuvant treatment of breast cancer [10].
It is important to note that a large number and variety of compounds derived from medicinal plants have antineoplastic activity, such as vinca alkaloids, taxanes, epipodophyllotoxins, and camptothecin derivatives, among others. Antineoplastic drugs derived from plants are mostly characterized as antimitotic agents, which induce the death of cancer cells while they are in mitosis [11,12]. For this reason, they also affect healthy cells and cause several unwanted side effects in most chemotherapies. However, their cytotoxic effects are also associated with mechanisms such as free radical scavenging, the reduction of tumor angiogenesis, and activation of multiple signaling pathways mediated by membrane receptors, kinases, transcription factors, microRNAs, cyclins, and caspases [13,14].
Hormonal therapy is another form of systemic therapy that is indicated as the treatment of choice for hormone-dependent (estrogen and/or progesterone receptor) breast cancer [2]. It is given over a long period of time, which increases the risk of significant side effects. Immunotherapy differs from other treatments that not only destroy malignant cells but also significantly affect healthy tissue [15]. The goal of immunotherapy is to stimulate the patient’s immune system to recognize and selectively destroy malignant tumor cells. It is usually combined with other therapies to achieve better results, as it provides proven benefits without increasing side effects [16]. Currently, there are several forms of immunotherapy: (a) active immunotherapy (vaccines), in which the tumor proteins are injected into the patient; (b) adoptive cell transfer, in which cells from the patient’s immune system are used to expand them and help them respond; (c) regulatory agents, such as cytokines, which are injected into the patient to enhance the immune response; and (d) monoclonal antibodies, which are injected to bind to specific receptors in the patient’s body [15,17].
One of the major problems in the pharmacological treatment of breast cancer, whether chemotherapy, hormone therapy, or immunotherapy, is the lack of specificity of the treatments for diseased or healthy tissues. One way to reduce the non-specificity of drug treatments on certain tissues is to couple them to controlled and specific release systems, among which researchers can highlight nanoparticles and hydrogels composed of polymeric matrices and the conjugation of two or more elements in a therapeutic option whose objective is to increase their safety [18,19,20]. On the other hand, the fact that genetic alterations occurring in tumor cells or in their microenvironment affect the efficacy of drug therapy makes gene therapy an increasingly considered a relevant tool for this disease [21].
2. Herbal Treatments for Breast Cancer
Many therapies are used to fight and eradicate breast cancer: surgery, radiation therapy, chemotherapy, and hormone therapy [10]. However, aggressive treatments such as radiation and chemotherapy can have adverse effects on the body because they do not only target tumor cells and can negatively affect patients. One of these side effects, which significantly influences the success of the treatment, is the reduction of the immune system activity, leading to immunosuppression, which promotes the development and progression of tumors [22].
It is important to note that some types of breast cancer are resistant to conventional therapies, which poses a significant challenge in eradicating malignant cells [22]. Due to the aforementioned drawbacks and similar concerns, it has become necessary to explore new therapeutic options to treat breast cancer effectively and, at the same time to be less aggressive to the body, causing minimal or no side effects, less toxicity, and less likelihood of inducing treatment resistance to improve the patient’s quality of life [22,23].
The phytochemical components of plants are a therapeutic option whose properties have been known for a long time; however, with the appearance of synthesized chemical compounds with anti-cancer activity, the use of plants decreased. A few years ago, the use of natural remedies to treat various diseases became relevant again due to their preventive and therapeutic properties. Plants possess phytochemical components with antioxidant and anti-inflammatory properties, mainly coumarins, flavonoids, alkaloids, and terpenoids. Several studies have demonstrated that bioactive compounds from plants can exert anti-tumor activity through various signaling pathways, such as apoptosis, autophagy, modification of the tumor microenvironment, cell arrest, and the suppression of angiogenesis. Selected plants are included in Table 1 because they have many in vitro studies on different types of breast cancer cell lines and in vivo studies in different mouse models of breast tumors. Studies have shown that the structural parts of plants, such as leaves, fruits, and roots, contain chemical compounds with great potential. These compounds have been extracted using various methods and used as whole extracts or fractions to test their effectiveness in inhibiting the growth of various types of breast cancer that affect women worldwide. Certain compounds have been extensively studied and exhibit anti-tumor properties, as they have both anti-proliferative and anti-apoptotic effects. In addition, they can modulate the immune system by activating immune cells and cytokines that can attack or inhibit tumor cell growth (Figure 1) [24,25,26,27,28].
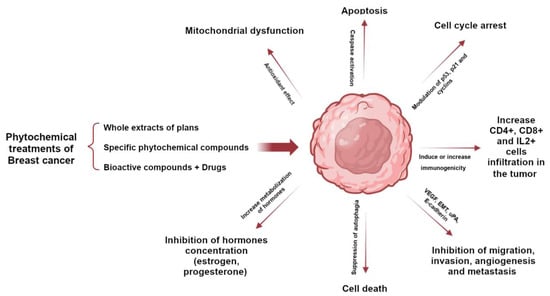
Figure 1. Role of phytochemicals in breast cancer to induce tumor cell death through activation or inhibition of signaling pathways.
Table 1. Anti-cancer effects of plants and extracts on breast cancer cell lines and experimental models. LD, lethal dose 50; mg, milligram; kg, kilogram; µg, microgram; ml, milliliter; Rg3, ginsenoside Rg3; SD, Sprague Dawley.
| Ingredients/Extracts | Experimental Setting/Model | Antitumoral Activity/Mechanism | Toxicity/Tolerability | Reference | |
|---|---|---|---|---|---|
| Ginseng species | Ginsenosides | MCF-7 MDA-MB-231 |
Anti-proliferative effect Induction of apoptosis |
Rg3: LD50 > 800 mg/kg in SD rats LD50 > 1600 mg/kg in mice Does not cause toxicity or mortality |
[29,30,31] |
| Allium sativum (Garlic) |
Diallyl disulfide | MDA-MB-468 | Induction of apoptosis Inhibit resistant cell proliferation cell cycle |
Oral administration > 1600 mg/kg in mice and no toxicity or mortality was observed | [32,33] |
| Curcuma longa | Curcumin | BT-483 MCF-7 MDA-MB-231 |
Anti-proliferative effect Induction of apoptosis |
LD50 500 mg/kg by intraperitoneal administration in the rat LD50 > 1000 mg/kg by the oral route in the rat Oral administration to mice and rats at a high dose of 5000 mg/kg did not cause toxicity or mortality |
[34,35] |
| Arctium lappa (Greater burdock) |
Arctigenin | MDA-MB-231 MCF-7 |
Induction of apoptosis Anti-metastatic effect |
LD50 > 5000 mg/kg in Wistar rats by the oral route Repeated administration (300 mg/kg) had some toxic effects on the lungs and small intestine |
[36,37,38] |
| Nigella sativa | Thymoquinone | T-47D MDA-MB-468 MCF-7 MDA-MB-231 |
Induction of apoptosis Anti-metastatic effect The study showed a protective effect against the development of breast cancer in a rat model |
LD50 104.7 mg/kg in mice by intraperitoneal administration and 870.9 mg/kg by oral administration LD50 57.5 mg/kg by intraperitoneal administration and 794.3 mg/kg by oral administration Safety with oral administration higher than with intraperitoneal administration |
[39,40,41] |
| Camellia sinensis (Green tea) |
Epicatechin gallate (EGCG) | MCF-7 MDA-MB-231 MCF-10A 4T1 |
Modulate p53 levels Reduce breast cancer cell viability and migration Induce apoptosis |
LD50 2828.43 mg/kg in mice by the oral route LD50 186.8 mg/kg in rats by oral administration was safe 1868 mg/kg showed toxic effects and mortality |
[42,43,44] |
| Echinacea | Echinacoside | BT-549 MDA-MB-231 MDA-MB-468 MDA-MB-231 xenograft model in vivo |
Inhibition of cell proliferation Reduce tumor growth |
2500 mg/kg in mice by the intraperitoneal route 30 g/kg in mice by the oral route Toxicity and mortality not reported at 15 g/kg in the rat |
[45,46] |
| Linum usitatissimum L. (Flaxseed) |
Lignans | T-47D MCF-7 MDA-MB-231 Athymic mice inoculated with human MCF-7 cancer cells |
Inhibit cell proliferation and induce apoptosis Reduce tumor growth |
>15 g/kg in SD rats by oral administration Excessive ingestion may result in abnormal hematopoietic and hepatic functions |
[47,48,49,50] |
| Moringa oleifera leaf | Polyphenols (Mopp) were encapsulated with phytosomes |
4T1 MCF-7 |
Anti-proliferative effect on cancer cells in vitro | LD50 > 2000 mg/kg in female albino Swiss by oral administration Consumption safe < 70 g/day |
[51,52,53] |
| Strobilanthes crispus | Bioactive compounds identified were lutein, beta sitosterol, stigmasterol, 131-hydroxy-132-oxo-pheophytin a, campesterol, pheophytin a, and 132-hydroxy-pheophytin a. in fraction 3 |
4T1 MDA-MB-231 Mammary tumor induction in BALB/c mice |
Inhibit migration, invasion, and metastasis Enhance immune system activity Increase MHC class I and MHC class II molecules in vitro Increase CD4+, CD8+ and IL-2+ cells infiltration into the breast tumor microenvironment |
LD50 > 600 mg/kg in SD rats by oral administration Consumption safe < 3–4 g/day |
[54,55,56,57,58] |
| Decalepis arayalpathra | 2-hydroxy 4-methylbenzaldehyde (2H4MB) | MDA-MB-231 MCF-7 |
Antioxidant and anti-cancer activities: induction of apoptosis by loss of mitochondrial membrane potential and cell cycle arrest | Not reported | [59,60] |
| Mangifera indica (Mango) | Kernel, bark, leaves, peels, and pulp | BT-474 | Induce apoptosis and cell cycle arrest. Reduce proliferation, growth, migration and invasion Reduce tumor weight and volume |
LD50 > 2000 mg/Kg 90-day repeated dose oral toxicity study in Wistar Han rats | [61,62,63,64] |
| Phytochemicals (mangiferin, norathyriol, gallotannins, gallic acid, pyrogallol, methyl gallate, and quercetin) | MCF10DCIS MDA-MB-231 BT-549 T47D MCF-7 Mice bearing tumor cell line (MCF10DCIS, MDA-MB-231 and BT-549) xenografts (female) Athymic nude mice xenografted with MCF10DCIS |
||||
| Scorzonera hispanica (Asteraceae) seeds | SH1, SH4 and SH11 (apigenin, derivatives of p-coumaric and caffeic acids, fatty acids- and 3,4-dimethoxycinnamate) |
MDA-MB-231 MCF-7 |
Induce apoptosis Exhibit the ability to inhibit the expression of the pro-survival protein BCL-2 and increase the expression of the apoptosis-accelerating protein Bax |
IC50 95 μg/mL was determined for cytotoxicity against the P388 murine leukemia cells | [65,66] |
| Cyclopia species | SM6Met, cup of tea (CoT) and P104 | MCF-7 T47D |
Regulation of the estrogen receptor alpha and beta subtypes occurs through various mechanisms, including transcriptional, translational, and proteasomal degradation | IC50 of 88.4 μg/mL for the viability of C3A cells | [67,68] |
| Garcinia species | Mangostin, Cambogin, Gambogic Acid, Garcinol, Griffipavixanthone, Friedolanostane triterpenoid, Hexane, and Neobractatin, 7-Epiclusianone, xanthochymol-guttiferone E, and isoxanthochymol-cycloxanthochymol |
T47D MCF-7 MDA-MB-231 BJMC3879 4T1 AU-565 BT-483 MDA-MB-435 |
Induce apoptosis Inhibit proliferation and metastasis |
LD50 > 5g/kg in a single dose 1000 mg/kg daily for 28 days caused no mortality |
[69,70] |
Most of the active ingredients of phytopharmaceuticals are poorly soluble in water, which limits their bioavailability, their ability to cross biological membranes, and thus their effective use in the treatment of various diseases, including cancer. The stability of phytopharmaceuticals and their low specificity are also relevant aspects in their clinical application [71].
On the other hand, the techniques chosen for the extraction of active compounds from plant species (phytopharmaceuticals) depend on the chemical nature and physicochemical properties of these compounds, in addition to the characteristics of the plant material to be worked with. It is important to consider that these compounds are immersed in a complex matrix that includes plant structures that make them difficult to obtain, in addition to biomolecules with different functional groups and polarities [72].
Once the extraction process is complete, the compound of interest will most likely be found as part of a mixture of different compounds that were also extracted with it. It is possible to perform a bioassay-guided fractionation of the extract by separating its fractions. The resulting fractions are evaluated by a biological assay to identify those that induce the desired biological activity. This fractionation–bioassay process is repeated until a compound is obtained that is as pure as possible or until a biological activity is obtained at low concentrations of the evaluated fractions with a sufficient yield to perform all required assays [71,72].
The potential of phytopharmaceuticals has already been widely demonstrated in the clinic. Medicinal plants, or products derived from medicinal plants, including pure molecules, extracts, or fractions of extracts, have historically been the major source of anti-cancer drugs approved for use in humans; phytopharmacology has resulted in highly successful drugs in the clinic. Phytochemicals synthesized by plants with anti-tumor activity in breast cancer have a wider margin of safety. They can act synergistically with chemotherapeutic drugs to increase the efficacy of treatment and sensitize tumor cells to chemotherapy with the advantages of reducing the dose of the chemotherapy and reducing the adverse and toxic effects of chemotherapy (Table 2) [73,74], either by interacting pharmacologically (pure compounds administered separately) or by targeting and delivering of both compounds via nanocarriers.
Table 2. Research on the potential benefits of combining herbal treatments with established chemotherapy drugs for breast cancer.
| Plant | Phytochemical Anti-Cancer | Drug | References |
|---|---|---|---|
| Papaver somniferum (Opium poppy) |
Noscapine | Docetaxel | [75,76] |
| Nigella sativa | Thymoquinone | Cyclophosphamide Doxorubicin Tamoxifen Paclitaxel |
[77,78,79,80] |
| Vaccinium myrtillus L. (Blueberries) |
Resveratrol | Raloxifene Doxorrubicin Paclitaxel |
[81,82,83] |
| Curcuma longa | Curcumin | Doxorubicin 5-fluorouracil Paclitaxel |
[84,85,86] |
| Zingiber officinale (Ginger) |
Gingerol | Doxorubicin | [87] |
| Ginseng species | Ginsenoside Rg3 | Paclitaxel | [88] |
| Camellia sinensis (Green tea) |
Epigallocatechin gallate (EGCG) and quercetin |
Tamoxifen | [89] |
| Echinacea | Hexane fractions of Echinacea purpurea containing cynarin |
Paclitaxel | [90] |
| Arctium lappa on | Arctigenin | Doxorubicin | [91] |
This entry is adapted from the peer-reviewed paper 10.3390/futurepharmacol3040043
This entry is offline, you can click here to edit this entry!
