Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Niosomes, transfersomes, and ethosomes are nanometric vesicular structures that allow drug encapsulation, protecting them from degradation, and increasing their solubility, permeability, brain targeting, and bioavailability.
- Alzheimer’s disease
- Parkinson’s
- schizophrenia
- transfersomes
1. High Prevalence Brain Disorders: Current Treatments and Their Limitations
Brain disorders represent a significant global burden, being estimated to affect over 1 billion people worldwide, being major causes of disability, and even increasing mortality, especially in middle- and high-income countries [1,2]. These illnesses contribute to the global disease burden quite significantly, and more than all other non-communicable diseases, such as cardiovascular diseases, or even cancer [3,4,5]. As global awareness and access to information expand, so do the number of diagnoses, but the increase in average human life expectancy also has an important impact on the estimated incidence of these diseases, since brain functions deteriorate as this organ ages [3,5]. Although psychiatric and neurodegenerative disorders are usually classified separately, they often exist simultaneously, which not only contributes to polymedication and, consequently, decreased patient treatment compliance, but also results in a more difficult diagnosis, since some symptoms tend to overlap [1,4,5]. Some of the most prevalent and disabling diseases with a brain etiology are schizophrenia, bipolar disorder, depression, anxiety disorders, Alzheimer’s disease, and Parkinson’s disease (Figure 1).
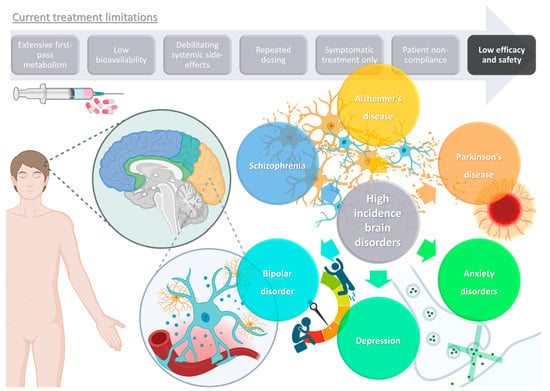
Figure 1. Schematic representation of high-incidence brain disorders and limitations of current treatments (produced with Biorender).
Schizophrenia is a psychiatric disorder that is usually developed in early life, due to abnormal brain development, including structural and functional changes in the regions of the medial temporal and prefrontal lobes, which are brain regions involved in declarative and working memory regulation [6,7,8]. These alterations have led to the disease being characterized by different behavioral and cognitive symptoms, which are classified as either positive (related to experiences that are “an addition to reality”), such as delusions, hallucinations, disorganization, and erratic thought patterns, or negative (related with loss of certain abilities), including slow movements, general lack of motivation, and withdrawment [7,9,10]. Patients tend to lose their capability to perform simple daily tasks or be in socially demanding situations, which at many times leads to both occupational and social decline [10,11]. Schizophrenia has a strong genetic component, being passed on generationally, and its pathophysiology is generally connected to how the brain processes information, including changes in specific brain cell populations and the communication between them, and having been associated with neurochemical imbalances in the levels of dopamine, γ-aminobutyric acid (GABA), glutamate, and serotonin [6,9,10].
Similarly, bipolar disorders are also one of the leading disability causes among young people, being closely related to schizophrenia in the way that they also lead to functional and cognitive impairments [14,15,16]. Early diagnoses are difficult to achieve, since some symptoms overlap with other disorders, such as mood swings (schizophrenia) or depressive episodes (depression) [15,16,17]. Bipolar disorders can either be classified as type I, being mostly characterized by the existence manic episodes (uncontrollable thoughts, restlessness or hyperactivity, obsessive compulsive behavior, exaggerated emotions, and extreme mood changes, including magnified irritability or happiness), or type II, being characterized by hypomanic episodes (a less severe form of mania, with similar symptoms as manic episodes, but in a less intense manner) associated with depressive features [14,15,18]. Gene–environment interactions are believed to explain their etiology the best, having a strong genetic component (approximately 70% heritability), but also being commonly related to traumatizing events, especially those occurring in early life (adverse environmental exposures, such as childhood maltreatment) [14,17]. Its pathogenesis is believed to be related to disturbances in monoaminergic signaling, neuronal–glial plasticity, cellular metabolic pathways, inflammatory responses, and mitochondrial function [15,16]. Pharmacological treatment often includes antipsychotic (quetiapine, lurasidone, and olanzapine), antidepressant (amitriptyline, paroxetine, and bupropion), or even some antiepileptic drugs (carbamazepine, lamotrigine, and valproic acid), although lithium continues to be one of the most effective therapies, due to its general mood-stabilizing properties, with specific antidepressant and antimanic effects due to its role in the reduction of dopamine and glutamate and increase of GABA neurotransmission [15,19,20,21].
On the other hand, depression has such a high incidence that it not only affects the patient’s quality of life severely, but it also has a heavy impact on society in general, due to its associated healthcare costs and lowering of work productivity [22,23]. Even though there are several subtypes (major depression, persistent depressive disorder, depression associated with bipolar disorder, postpartum depression, etc.), with different symptoms (or differences in symptom intensity or frequency), overall clinically depressed individuals usually experience loss of interest and/or anhedonia, low self-esteem that comes with feelings of worthlessness, hopelessness, or guilt, mood swings or overall depressed mood, exaggerated loss of energy or general fatigue, psychomotor and sleep alterations, appetite and/or weight alterations, and cognitive difficulties, such as trouble thinking or concentrating, or general indecisiveness [24,25,26].
Anxiety disorders are another type of very common psychiatric disorders, involving a brain circuit’s dysfunction in response to perceived danger [30,31]. Relevant subtypes include generalized anxiety disorder, social anxiety disorder, phobias, panic disorder, among others [30,32,33]. These disorders often come hand-in-hand with depression, and similarly to depression, anxiety disorders lead to an overall reduction of the patient’s quality of life and productivity, being associated with high health and social costs [30,31,34]. Additionally, the comorbidity of anxiety with depression usually originates from more severe symptoms and a higher difficulty in attaining an effective treatment [30]. Although some of the main symptoms overlap with those also frequently present in depression, such as trouble sleeping, anxiety disorders are usually characterized by a chronically intense nervousness or restlessness, easy irritability, worry and fear, fatigue, trouble concentrating, dizziness, stomach discomfort, headache or pain located in other places of the body, increased muscular tension, changes in heartbeat, sweating, and/or a light feeling of “needles” on the skin’s surface [30,32,33]. Non-pharmacological treatment includes psychotherapy (cognitive behavioral therapy), and pharmacological treatment includes selective serotonin reuptake inhibitors (e.g., sertraline, escitalopram, and fluoxetine), serotonin and norepinephrine reuptake inhibitors (e.g., duloxetine and venlafaxine), or the commonly used benzodiazepines (alprazolam, diazepam, and midazolam), which act as GABA receptor agonists, especially for type A [31,32,33].
In contrast, Alzheimer’s disease is a neurodegenerative disease, known to be a severely impairing illness, characterized by a slow but progressive loss of brain function, including cognitive decline, with memory and thinking ability failure, which often progresses to dementia [36,37,38]. It is a major cause of disability, also being connected to increased mortality, with the person developing high levels of dependence, since aside from experiencing difficulty in remembering things, which worsens with time, patients also tend to lose their ability to communicate, as well as the awareness of their surroundings [36,38,39].
Parkinson’s disease also has a neurodegenerative nature, with patients usually experiencing severe and gradually intensified movement impairments [49,50,51]. Common symptoms include tremors, postural instability, akinesia, bradykinesia, and/or rigidity, which will make the patient progressively less capable of performing even the simplest daily tasks on their own [49,51,52]. Nevertheless, non-motor symptomatology also plays a clinically significant role, including drowsiness and sleep disorders, constipation, impaired olfaction, or even depression and/or psychosis [50,52,53]. With genetics also playing an important role, and having the most incidence in the elderly, its pathophysiology mostly relates to neuronal loss, more specifically of dopaminergic neurons, in certain brain areas, such as the substantia nigra [50,51,52]. It is also characterized by the development of Lewy bodies, containing an accumulation of the protein α-synuclein [50,52,53]. The most commonly used pharmacological therapy is levodopa, which is converted into dopamine via enzymatic degradation after undergoing uptake by neuronal cells in the brain [49,51,53]. Dopamine agonists are also used, with ergot dopamine agonists mainly acting on D2, D3, and D4 receptors (e.g., cabergoline, bromocriptine, and pergolide), and with non-ergot dopamine agonists acting more selectively on D2 or D3 receptors (e.g., ropinirole and pramipexole), with anticholinergics (e.g., benzhexol, orphenadrine, and benztropine), catechol-O-methyltransferase inhibitors (e.g., entacapone, tolcapone, or opicapone), monoamine oxidase-B inhibitors (e.g., selegiline and rasagiline), and amantadine (N-methyl-D-aspartate-glutamate and cholinergic muscarinic receptor inhibitors) also being commonly prescribed [54,55,56]. Non-pharmacological treatments, such as physical exercise and physiotherapy (involving endurance, balance, strength, and coordination), along with cognitive training (memory and logical thinking), are also required in order to slow down the disease’s progression [57,58].
Despite having some different characteristics, all these disorders tend to have a chronic nature, lasting for many years or even a lifetime, and hence have a very significant impact not only on the individuals themselves, but also in the society in which they are inserted. Additionally, as mentioned, aside from a lack of therapeutic efficacy and the need for repeated dosing regimens, and with many existing treatments only being effective for the disease’s symptoms, hence not being a “true cure”, most medications lead to a wide variety of systemic side effects, some of which are quite serious and disabling, with a highlight on sedation (benzodiazepines), weight gain that can culminate into obesity and associated problems (antidepressants and antipsychotics), neuroleptic malignant syndrome (antipsychotics and antiparkinsonian agents), cerebrovascular accidents (antidepressants and antipsychotics), induced parkinsonism (anti-Alzheimer agents), and even suicidal ideation (antidepressants and anxiolytics) [59,60,61,62,63], and, of course, taking more than one drug simultaneously will increase the propensity, intensity, and/or frequency of such side effects, which unfortunately is not a very unlikely scenario, since psychiatric diseases tend to coexist, either due to similar pathophysiology and/or causes, medication side effects, or even with one psychiatric illness leading to another (as is the case of depressive and anxiety disorders being commonly diagnosed together) [59,60,61,62].
Furthermore, aside from efficacy and safety issues, most drug molecules also have characteristics that will hinder their bioavailability, such as high first-pass metabolism, low permeation through the biological barriers (especially the blood–brain barrier (BBB)), high plasma protein binding, considerable P-glycoprotein efflux, and low water solubility, which will make it difficult to both formulate them at high strength and make them reach the intended therapeutic site at concentrations required for the therapeutic effect to occur (in this case, the brain) [64,65,66,67,68]. Nevertheless, although these problems are not easily overcome using conventional formulations, nanotechnology can be the answer.
2. Nanosystems as Non-Conventional Forms of Treatment for Increased Efficacy and Safety
Nanotechnology is the science that manipulates matter at the nanometric scale, and over the years it has proved to be advantageous when applied to a variety of fields, such as engineering, food, cosmetic science, and medicine, both in diagnostics and therapeutics [69,70,71,72]. In pharmaceutical nanotechnology, the development of nanosystems (structures with sizes between 1 nm and 1000 nm) for drug molecule encapsulation has proven to be beneficial in the treatment of a wide number of diseases, including cardiovascular, oncological, and neurological diseases, among many others [69,73,74,75,76].
Although nanosystems can be divided into categories according to their composition, amongst other specific characteristics, in general, all of them offer a means to protect drug molecules against chemical or metabolic degradation, due to encapsulation within structures that provide a barrier to the outside environment [77,78,79]. Additionally, they can also increase drug permeation through biological barriers, and when functionalized with specific moieties, which are molecules that will be inserted onto the surface of the nanoparticles and that will bind to specific cell surface receptors on target tissues, such as proteins and peptides (lectin, transferrin, lactoferrin, apolipoprotein E, etc.), or smaller molecules (polyethylene glycol, folic acid, galactose, mannose, etc.), will provide active drug transport into these target cells and tissues, and, hence, drug targeting, thus allowing an increased bioavailability in these tissues and decreased systemic drug distribution, leading to potentially safer and more effective therapies [77,80].
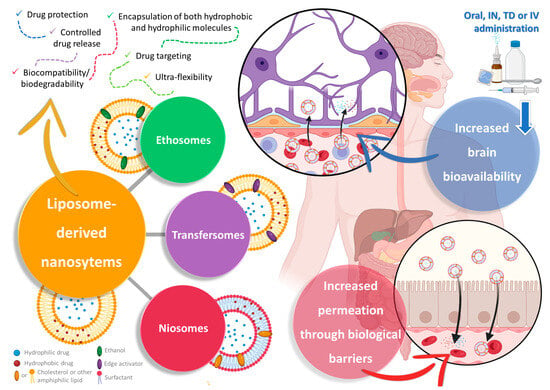
Liposomes are a specific type of spherical vesicular nanosystem, characterized by having one or more phospholipid bilayers surrounding an aqueous core (Figure 2). This structure gives this type of nanosystem the important advantage of being capable of encapsulating both hydrophobic and hydrophilic molecules in the membrane (hydrophobic nature) or in the core (aqueous nature), respectively, either separately or simultaneously [81,82,83]. Given this very advantageous versatility, and with the added advantages of being biocompatible and biodegradable, having controlled drug release, and leading to an overall increased drug absorption and, consequently, bioavailability, liposomes have received a substantial amount of attention from both academic- and industrial-based research [81,84,85]. Additionally, due to favorable results in targeting and accumulation in tumor tissues, a few liposomal formulations have already reached the pharmaceutical market for cancer treatment [81,83,84].

Figure 2. Schematic representation of liposome-derived vesicular nanosystems, namely ethosomes, transfersomes, and niosomes, and their respective composition and advantages in drug delivery (produced with Biorender). IN—intranasal; IV—intravenous; TD—transdermal.
Ethosomes, just like the name implies, have ethanol in their composition at high concentrations (from 20% to 50%), as well as phospholipids (such as cholesterol, phosphatidylcholine, and phosphatidylethanolamine) [84,86,87]. Common preparation methods include the cold method, the hot method, the classic mechanical dispersion method, and the transmembrane pH gradient method [86,87]. Nevertheless, despite having ethanol in their composition, and just like liposomes, ethosomes have been reported to be biocompatible, biodegradable, and generally non-toxic [84,88,89].
On the other hand, transfersomes (sometimes also called “transferosomes”) are likewise bilayered vesicles, similar to ethosomes in the way that they are altered liposomes, but instead of ethanol they have the addition of an edge activator, usually surfactants, such as Tweens, Spans, or sodium deoxycholate, to the lipid bilayer, usually made of phosphatidylcholine or lecithin [92,93,94]. The addition of these softening compounds to the bilayer will allow these nanosystems to have ultra-flexibility, due to elasticity and deformability capacity, which will make them able of deforming and squeezing through and across biological membranes, including through pores substantially smaller than their own size, such as intercellular gaps, while remaining intact, hence increasing drug permeation [84,92,95]. These edge activators also have the capability of solubilizing or fluidizing the cells’ wall lipid components, allowing for a higher cellular uptake [93,95]. Aside from having reported higher drug permeation and deposition, these vesicles have also been reported to have a higher drug entrapment efficiency than their ancestor’s liposomes, higher stability (prevention of drug degradation via temperature, oxidation, or even light), and a relevant controlled release capacity [84,92,94].
Finally, niosomes, the most recently developed technology to overcome the disadvantages of liposomes as drug delivery systems, are likewise nanometric lamellar structures, mainly composed of a lipid (mostly cholesterol) and a non-ionic surfactant [96,97,98]. Nevertheless, unlike what happens in liposomes, in niosomes, cholesterol has a support function, shaping the vesicles and providing stiffness to their structure, while the main constituent of the bilayer is the surfactant, namely amphiphilic non-ionic surfactants, such as sorbitan esters (Span), polysorbates (Tween), or others [84,96,97]. Niosomes can be generally classified as either small unilamellar (one bilayer, between 10 nm and 100 nm), large unilamellar (one bilayer, larger than 100 nm), or multilamellar (more than one bilayer, larger than 50 nm) [96,98,99]. While smaller unilamellar vesicles have the advantages of being typically more stable (high thermodynamic instability) and easier to obtain, larger vesicles, and/or with a higher number of layers, have the ability to encapsulate a higher amount and/or wider variety of drugs (more than two drugs simultaneously, and/higher amounts of each one) [96,99]. Common preparation methods include the thin-film hydration method, the ether injection method, the reverse-phase evaporation method, the microfluidization method, the transmembrane pH gradient method, the bubble method, the supercritical carbon dioxide fluid method, the heating method, or the ball milling method, all typically followed by sonication whenever necessary to reduce particle size and increase its homogeneity [84,96,97]. Niosomes have the advantages of reportedly being more stable, having a higher entrapment efficiency, and having a more controlled drug release capacity and targeted delivery capability than liposomes [96,98].
3. Liposome-Derived Nanosystems: Ethosomes, Transfersomes, and Niosomes for Brain Drug Delivery
3.1. Ethosomes
Ligustrazine is a natural-derived compound used in traditional Chinese medicine for the treatment of several different cardiovascular and cerebrovascular diseases. Recently, some studies have shown that this alkaloid, extracted from Ligusticum chuanxiong Hort (Haoben Chuanxiong), effectively has neuroprotective effects, related to its marked antioxidant, anti-inflammatory, anti-apoptotic, calcium overload inhibition, and enhanced hippocampal cholinergic system function effects, resulting in learning and cognitive function improvements. Nevertheless, when administered orally, this compound leads to variable drug absorption, low bioavailability, and a short elimination half-life due to extensive hepatic first-pass metabolism, which makes frequent administration necessary. Furthermore, its intravenous administration, aside from having the general disadvantages associated with invasive administrations, requires infusion for a long period of time, leading to reduced patient compliance and both local and systemic side effects [106,117,118,119].
3.2. Transfersomes
Curcumin is another natural-derived and thoroughly studied compound, extracted from the rhizomes of Curcuma longa L. It has been described to have substantial anti-inflammatory and antioxidant effects, which are mostly connected to the polyphenols that are part of its composition. It has also been proven to have neuroprotective properties in Alzheimer’s disease, namely by targeting neurotrophins and cellular processes connected to cytokine production [107,126,127,128]. On the other hand, berberine, a natural-occurring alkaloid extracted from Berberis species, which has long been used in traditional Chinese medicine, has also been demonstrated to have neuroprotective effects, namely through inhibition of the apoptosis-inducing Akt/ERK1/2 signaling pathway, c-Jun N-terminal kinase pathway, and GSK-3β and caspase-3 activity [107,129,130,131]. Nevertheless, despite the strong suggestion of beneficial effects in the treatment of neurodegenerative diseases, both compounds have limited efficacy due to low solubility and low bioavailability. To address these issues, Mishra et al. [107] decided to simultaneously formulate both molecules in transfersomes, for intranasal administration, for the treatment of Alzheimer’s disease. Aside from being non-invasive, easily appliable, and a good alternative to the oral route when it is not available, intranasal administration has the major advantage of allowing for at least part of the drug to be transported directly from the nasal cavity to the brain, via neuronal pathways, a process known as nose-to-brain drug delivery [132,133]. This makes it ideal for brain diseases, since the drug will be transported to the intended therapeutic site of action without undergoing first-pass hepatic metabolism, and without having to pass through the BBB, which is known for having a very low level of permeability to most molecules [134,135]. Hence, intranasal delivery will not only allow an increase in brain drug bioavailability, but also a decrease in systemic drug distribution, making it both a more effective and safer therapeutic alternative, while also having a faster onset of action than other administration routes, making it ideal for managing emergency situations [136,137].
Recent studies have also proven that insulin can have a potentially beneficial role in the treatment of Alzheimer’s disease, leading to improved cognitive function, with neuroprotective effects being mainly due to action against oxidative stress, inflammation, and mitochondrial damage related to the PI3K/Akt and MAPK signaling pathways. Additionally, insulin has proven to increase glucose metabolism in brain cells, lead to the changing of Aβ oligomers’ ratio, and increasing brain high-energy phosphate content, all of which have an important role in the disease’s pathophysiology. Nevertheless, being a protein, it is hard to deliver insulin to the brain, especially due to its high molecular weight, leading to low permeability through the BBB, and also high susceptibility to degradation [108,138,139,140]. Hence, in order to deliver insulin with high efficacy to the brain, for the treatment of Alzheimer’s disease, Nojoki et al. [108] encapsulated it inside transfersomes for intranasal delivery. The nanosystems were produced via thin-film hydration and were composed of soy lecithin (a phospholipid) and Tween 80 (an edge activator). These transfersomes had a particle size, PDI, ZP, and EE of 95.2 ± 19.0 nm, 0.265, −3.5 mV, and 69.6 ± 1.2%, respectively. Then, a variation of these transfersomes was made by modifying their surface with chitosan. The chitosan-coated transfersomes showed a slightly bigger particle size (137.9 ± 28.2 nm), but smaller PDI (PDI 0.20), and a similar EE (65.1 ± 0.9%). The coated transfersomes also presented a higher and positive ZP (+23.4 mV), suggesting that the coating with chitosan, a positively charged polymer, was indeed successful. The developed particles had a spherical shape and proved to be reasonably stable for up to 3 months, under storage at 4 °C and 25 °C, since the particle sizes did not change significantly, hence suggesting that no aggregation phenomenon occurred, although there was a reduction in encapsulation efficiency.
Aripiprazole is another drug molecule approved for the treatment of diseases with a brain etiology, namely as a main therapy for schizophrenia and bipolar disorders, and as an adjuvant therapy for major depressive disorders. It is characteristically categorized as an atypical antipsychotic drug, acting as dopamine D2 and D3 and serotonin 5-HT1A receptor partial agonists, and as a serotonin 5-HT2A receptor antagonist. Aripiprazole is mainly administered via the oral and parenteral routes, but due to lack of brain selectivity, these formulations lead to several systemic side effects, some of them being quite severe, such as hypotension, somnolence, akathisia, tremors, or neuroleptic malignant syndrome. Aside from these safety issues, which directly affect patient compliance, being a hydrophobic drug not only leads to the difficulty of formulating aripiprazole with high strength but also results in a high variability of blood levels and, consequently, a variable and unpredictable therapeutic response. This drug is also a P-gp substrate, which limits its entry into the brain due to BBB efflux, limiting the amount of drug that is capable of reaching the intended therapeutic site of action [144,145,146,147].
On the other hand, asenapine, an atypical antipsychotic drug which mainly acts on dopamine D2 and serotonin 5-HT2A receptors as an antagonist, has low oral bioavailability, mainly due to liver and gut metabolism [110,148,149,150]. Hence, to solve this issue, Shreya et al. [110] encapsulated asenapine in transfersomes, for transdermal delivery, for the treatment of schizophrenia and bipolar disorder. The vesicles were once more prepared via thin-film hydration, followed by sonication, and were also made of soy phosphatidylcholine and sodium deoxycholate. Nevertheless, in this study, the transfersomes were then incorporated into an ethanolic Carbopol 934P gel, leading to a particle size of 126.0 nm, PDI of 0.232, ZP of −43.7 mV, and EE of 54.96%. Ex vivo permeation results (rat skin, Franz diffusion cells) showed that the transfersomal gel led to increased drug permeation, with an evident synergy existing between the used nanotechnological (transfersomes) and chemical (ethanol) permeation enhancement approaches. In the skin, ethanol will dissolve some of the stratum corneum’s lipids, transiently disrupting the skin barrier and leading to enhanced drug permeation.
3.3. Niosomes
As mentioned, asenapine is a dopamine and serotonin antagonist with low bioavailability. Hence, just like Shreya et al. [110], Singh et al. [111] developed vesicles to encapsulate this drug, for increased brain targeting, for the treatment of schizophrenia and bipolar disorder. Nevertheless, in this study, instead of transfersomes, niosomes were produced for oral administration. Oral drug administration is still the go-to administration route for most situations, due to being non-invasive, hence not bringing the patient pain or even discomfort, making it best for chronic therapies, and being easy to self-administer, leading to high patient compliance [151,152]. Additionally, it is possible to reach a prolonged therapeutic effect due to modification of the pharmaceutical oral forms due to controlled drug release, and the drug has access to a large area available for absorption to occur [153,154]. Furthermore, although the harsh environment of the gastrointestinal tract can lead to chemical and metabolic drug degradation, and even if the BBB has very low permeability to most drugs, nanoformulations can be designed to not only protect drugs, but also increase their permeation to the brain tissues [155,156].
Olanzapine is another atypical antipsychotic drug molecule, acting mainly on dopamine D2 and serotonin 5-HT2A receptors, which has low oral bioavailability due to extensive first-pass metabolism, also having low water solubility, making it a good candidate for encapsulation into niosomes [112,157,158,159]. This was exactly what Khallaf et al. [112] conducted, incorporating olanzapine into cholesterol and Span 80 niosomes for intranasal administration and for the treatment of schizophrenia and related psychotic disorders. The vesicles were produced via the thin-film hydration technique, followed by sonication. A variation of these niosomes was also made, by coating them with chitosan, due to this polymer’s bioadhesive properties, making it prone to interact with the nasal mucosa (more specifically with mucin), and also due to its additional ability to enhance drug permeation (transient opening of tight junctions). Both the uncoated and coated niosomes had a spherical shape and had a particle size of 241.30 nmand 250.1 ± 5.0 nm, an EE of 71.2% and 71.9%, and a viscosity of 3.1 ± 0.9 cP and 8.4 ± 1.2 cP, respectively. Hence, chitosan coating of the niosomes did not lead to significant changes in drug encapsulation, slightly increased the formulation’s viscosity, and also led to a small increase in particle size, which confirmed that the coating was in fact successful. The developed vesicles were also stable (6 months, 4 °C), showing only a small and statistically insignificant increase in particle size and decrease in EE. Ex vivo permeation assays (sheep nasal mucosa, Franz diffusion cells) revealed that the developed niosomes led to a better drug permeation through the nasal mucosa than a drug solution, probably in part due to the presence of a non-ionic surfactant in the vesicles’ membrane, an excipient type that is a known permeation enhancer.
Rivastigmine is an acetylcholinesterase and butyrylcholinesterase inhibitor used for the treatment of Alzheimer’s disease, leading to a reduction in the cognitive decline associated with cholinergic neuron degeneration. Nevertheless, it has extensive first-pass metabolism, leading to low oral bioavailability, and a short-half life, leading to the need for frequent administration [113,160,161,162]. On the other hand, N-Acetyl cysteine has also proven to have beneficial properties in neurodegenerative diseases, since it has been shown to increase glutathione levels, leading to an increased depletion of reactive oxygen species and, consequently, aiding in preventing the inflammation that is related with neuronal damage [113,163,164,165].
Moulahoum et al. [114] also attempted to develop an innovative treatment for Alzheimer’s disease, namely carnosine-loaded niosomes. Carnosine, which is another name for alanyl-L-histidine, is a natural-derived dipeptide, being present in all mammals, and can be found at its highest concentrations in the brain and muscle tissues, especially in the skeletal and cardiac muscles. Its potential for the treatment of neurodegenerative diseases arises from a substantial level of antioxidant activity (peroxyl radical, oxygen singlet and metal chelation, and related enzymatic regulation), with effective inhibition of advanced glycation end-products, inhibition of amyloid fibril formation, and suppression of β-amyloid accumulation, and protection of brain cells from its cytotoxic effects [114,166,167,168,169]. The niosomes were produced using the thin-film hydration method, followed by sonication, and were made of cholesterol and Span 60. Vesicle morphology was proven to be spherical, with a particle size of 560 ± 203 nm, and an EE of 32.4 ± 5%, with the developed formulation also proving to be stable for up to 30 days under refrigeration. The in vitro drug release assay (dialysis method) showed that the carnosine niosomes had a controlled drug release profile, and in vitro antiglycative and anti-aggregation assays proved that the developed vesicles led to decreased amyloid and fibrillation formation in a dose-dependent manner.
Other natural-derived molecules have also been proven to exhibit efficacy in the treatment of neurodegenerative diseases. Ginkgolide B, a diterpene extracted from Ginkgo biloba, has been proven to have potential for the treatment of Alzheimer’s disease, since by having an inhibiting role on the platelet-activating factor, it has been shown to protect neuronal cells that were damaged by Aβ accumulation from further harm. Additionally, this compound has also been shown to reduce the apoptosis induced by the Aβ peptide via the brain-derived neurotrophic factor mechanism, and to have reparative and protective effects on Aβ peptide-damaged mitochondria [115,170,171,172].
4. Liposome-Derived Vesicles: The Future for Brain Drug Delivery?
Given the very low permeability of the BBB to most drug molecules, delivering therapeutics to the brain becomes a significant challenge. Although the grand majority of marketed formulations are conventional formulations, decreased drug bioavailability at the target site and substantial drug distribution to other organs makes these preparations have low efficacy and safety. Nevertheless, as it has been made clear by the analyzed articles, scientists have developed novel alternatives in the nanosize scale, namely ethosomes, transfersomes, and niosomes, that are able to not only protect the drug molecules by encapsulating them, but also take them to the brain in a targeted manner, thereby increasing therapeutic outcomes in animal models. Although the mechanisms through which these vesicles are able to improve BBB penetration remain unclear, their nanosize and lipidic nature are thought to be relevant factors for increased permeation through any kind of biological barrier, since they are able to mimic these membranes’ composition, while being small enough to pass through them [90,182]. Additionally, the active transport of liposome-derived nanosystems to the brain, through transcytosis or receptor-mediated transport, has also been suggested, with binding to molecules such as glutathione or glucose possible playing a major role in vesicle translocation [90,183,184].
This entry is adapted from the peer-reviewed paper 10.3390/ph16101424
This entry is offline, you can click here to edit this entry!
