Acute myeloid leukemia (AML) is a clonal disease characterized by a broad spectrum of cytogenetic and molecular abnormalities that influence clinical outcomes. Despite the results achieved with the evolution of conventional chemotherapy and the inclusion of targeted therapies in the treatment of acute myeloid leukemia (AML), survival is still not satisfying, in particular in the setting of relapsed/refractory (R/R) disease or elderly/unfit patients. Among the most innovative therapeutic options, cellular therapy has shown great results in different hematological malignancies such as acute lymphoblastic leukemia and lymphomas, with several products already approved for clinical use.
1. Introduction
AML treatment for young and fit patients relies on intensive anthracyclines and cytarabine-based chemotherapy followed by allogenic hematopoietic stem cell transplantation (allo-HSCT) in selected patients. Treatment options in AML have recently expanded with the introduction of immune-conjugates, such as gemtuzumab-ozogamicin (GO) or tagraxofusp, and new targeted molecules; for example FLT3, IDH1, IDH2 and BCL2 inhibitors [
1]. However, the survival rate is still unsatisfying, especially in the setting of relapsed/refractory (R/R) disease or elderly/unfit patients.
Allo-HSCT consolidation is recommended for high-risk patients (i.e., those harboring adverse cytogenetic and molecular aberrations or those with positive minimal residual disease) in order to reduce the risk of relapse and prolong survival [
2]. The anti-leukemic effect of allo-HSCT mainly relies on the immunological cytolysis of residual leukemic cells mediated by the adoptively transferred donor’s immune cells (graft-versus-leukemia, GvL). In view of this, allo-HSCT can be considered the first successful clinical application of cellular immunotherapy [
3]. However, allo-HSCT is aggravated by the significant toxicities associated with the conditioning regimen, and by the risk of graft-versus-host disease (GvHD), therefore limiting the eligibility to allo-HSCT to only younger and fit patients. Recently, adoptive cellular therapy (i.e., CART-T cells) has shown considerable results in different hematological malignancies such as acute lymphoblastic leukemia and lymphomas, with several products already approved for clinical use [
4]. Despite the great interest in expanding the application of these new treatments to R/R AML, no product has been approved yet for this indication. This is partly due to the lack of a “pan-AML”-specific surface target, since many leukemia-associated antigens are commonly expressed on healthy myeloid cells, resulting in on-target-off-leukemia myeloablative effects [
5].
In this view, novel therapeutic approaches based on cell-based and adoptive immunotherapy represent an appealing strategy to overcome chemoresistance (i.e., in R/R or MRD positive patients) and alternative, less toxic consolidation strategies for patients ineligible for standard allo-HSCT.
2. T and NK Cells
The immune system plays an essential role in fighting against infections, inflammation, autoimmunity and cancer. Immune cells exert control in the early stages of cancer development, through the killing of malignant cells, thus hampering the chance of further tumor growth. The main immune cells involved in anti-tumor responses are cytotoxic CD8+ T cells (CTL) and Natural Killer (NK) cells, which are components of the adaptive and innate immune system, respectively (Figure 1A).
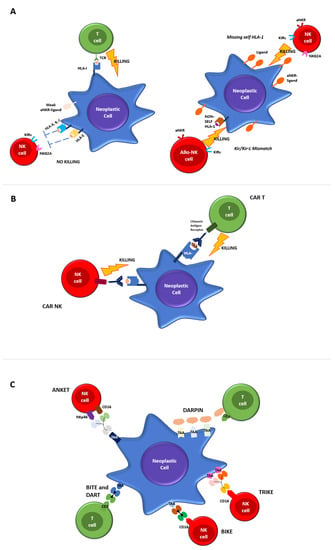
Figure 1. Mechanisms of T and NK-cell mediated killing. (A) Regulation of T and NK killing in physiological conditions. Cytotoxic T Lymphocytes express antigen-specific T cell receptors (TCRs) to recognize cognate peptides (antigens) bound to human leukocyte antigen (HLA) molecules on target cells. The binding of peptide/HLA-I complexes by TCR initiates T cell activation that converges in cytolytic activity. NK cell mediated-killing is regulated by complex interactions between inhibitory and activating receptors binding various ligands, including HLA-I, that prevents the killing of healthy autologous cells expressing appropriate levels of all self-HLA alleles and low/negative levels of ligands for activating receptors (left panel). The downregulation of HLA-I molecules on neoplastic cells induces NK-mediated killing by a “missing-self” recognition mechanism. NK cell-activating receptors are co-responsible for NK activation interacting with ligands overexpressed on neoplastic cells. Furthermore, NK cells kill neoplastic cells through the recognition of non-self HLA-I molecules (“KIR/KIR-ligand mismatch”), a mechanism of immunosurveillance active in the context of allogeneic-hematopoietic stem cell transplantation and in strategies of adoptive immunotherapies by alloreactive NK cells infusion (right panel). (B) CTLs and NK engineered with chimeric antigen receptors (CAR). The chimeric antigen receptor is made by a monoclonal antibody capable of binding to a specific identified target antigen, coupled with the activation of the intracellular proliferation signal domain. When the chimeric receptor binds to the antigen expressed in malignant cells, it stimulates the activation signal, leading to CAR-T cell activation and to the killing effects. (C) Novel platforms for T and NK cells engaging.
CTLs express antigen-specific T cell receptors (TCRs) to recognize cognate peptides (antigens) bound to human leukocyte antigen (HLA) molecules on target cells. The binding of peptide/HLA-I complexes by TCR initiates T cell activation that converges in cytolytic activity.
In contrast, NK cells lack specific antigen receptors but express a repertoire of inhibitory and activating receptors that bind various ligands, including HLA-I. A highly calibrated “education process” during the different steps of NK cells maturation allows the elimination of target cells with reduced or absent HLA-I molecules (“missing self”) while maintaining their tolerance towards cells expressing adequate levels of self-HLA. The main inhibitory NK receptors, KIRs and CD94/NKG2A, recognize classical (HLA-ABC) and non-classical (HLA-E) HLA-I molecules, respectively.
Activating NK receptors include a variety of non-HLA-specific receptors and co-receptors, which trigger NK cell stimulation via a straight interaction with ligands over/neo-expressed on malignant or virus-infected cells [
6].
Both CTL and NK cells, upon activation, undergo membrane reorganization and express various effector molecules to eliminate aberrant cells subjected to tumor transformation or infection by intracellular pathogens. Figure 1A provides a detailed overview of physiological T and NK cell-mediated killing.
T and NK cells mediated tumor killing occurs via direct cell cytotoxicity, the release of perforin and granzyme, NK cells antibody-dependent cellular cytotoxicity (ADCC) via engaging their receptor (CD16) or by apoptotic axis intermediated by the Fas ligand (FasL) or the TNF-related apoptosis-inducing ligand (TRAIL).
Indeed, a complex interplay between AML cells, bone marrow (BM) microenvironment and immune effector cells result in the dysregulation of innate and adaptive immune responses. A pivotal role in the induction of immune-tolerance with the enrichment of T regulatory cells in the context of the BM microenvironment is played by mesenchymal stromal cells, which support leukemic blasts and create a protective environment in response to pro-inflammatory stimuli [
10,
11].
A deeper understanding of the mechanisms regulating T and NK cell-mediated killing and AML interaction with the BM microenvironment paved the way for the development of effective cancer immunotherapies and post-transplant immunological interventions [
12,
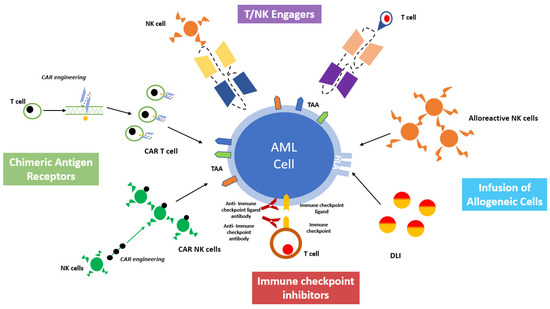
13]. The major aim of these approaches is to harness the immune system against AML, both by eliciting the cytotoxic effectors or by inhibiting the tolerogenic milieu of the BM microenvironment. A comprehensive overview of cell-based immunotherapy is provided in
Figure 2.
Figure 2. A comprehensive overview of cell-based immunotherapy strategies for acute myeloid leukemia treatment.
3. Principles of Adoptive Immunotherapy in Allogeneic Stem Cell Transplantation
3.1. Graft versus Leukemia Effect
Graft versus Leukemia (GvL) is defined as the anti-leukemic response mediated by donor’s immune cells in the context of allo-HSCT and has been the first established form of adoptive immunotherapy in cancer treatment. Donor’s T cells, which recognize residual leukemic cells through interactions with major histocompatibility (MHC) molecules on the surface of AML cells, are the major effector of adoptive immune-responses in allo-HSCT. The demonstration of a pivotal role of T cells mediating the GvL effect comes from past evidence of increased relapse rates observed using early T cell depleted graft platforms, as well as from the proven efficacy of post-transplant donor’s lymphocyte infusions (DLIs) in achieving disease control as both a prophylactic or pre-emptive strategy [
14].
However, the GvL effect is not limited to T cell activity. The anti-leukemic function of donor-derived alloreactive NK cells has been also specifically elucidated in the context of T-depleted haplo-identical HSCT (haplo-HSCT), a platform based on graft manipulation with a negative depletion of αβ-T and B cells, which allows the infusion of mature NK and γδ-T cells together with high doses of donor’s HSC [
15,
16]. In this setting, early reports from Ruggeri et al. showed that KIR ligand incompatibility in the graft-versus-host (GvH) direction (KIR-ligand mismatch) was an independent predictor of reduced relapse risk in a cohort of high-risk acute leukemia patients receiving haplo-HSCT [
17].
3.2. Donor’s Lymphocytes Infusion
Relapse after allo-HSCT still represents a major clinical challenge. Among mechanisms involved in leukemia relapse, CD4+/CD8+ T-cell exhaustion and the upregulation of inhibitor receptors (PD1 and Tim-3) on T-lymphocytes have been documented [
23]. Overall, in AML patients undergoing allo-HSCT, post-transplant prophylactic or pre-emptive donor lymphocyte infusions (DLIs) represent a simple and valuable therapeutic strategy to reduce the incidence of leukemia relapse, whereas its efficacy is limited in patients in overt relapse. DLIs act by repleting donor T cell pools and potentially reversing T cell exhaustion through an increase in terms of the function and proliferation of the CD8+ T cell resident in the recipient bone marrow [
24,
25].
The main toxicity associated with DLIs is the triggering of GvHD, which is strictly related to the dose of T cell infused. The risk of GvHD is significantly reduced without hampering efficacy when multiple, escalating doses of DLI, rather than single high-dose infusions, are administered [
26].
The first evidence of the anti-leukemic activity of DLIs was observed in Chronic Myeloid Leukemia (CML), in 1990 [
27]; several groups demonstrated that DLIs were able to induce complete hematologic and cytogenetic remission in up to 80% of chronic-phase CML patients relapsing after allo-HSCT [
28,
29]. However, results in blast-phase CML or AML patients relapsing after allo-HSCT were significantly inferior, with less than 25% of patients achieving short-term remissions.
Minimal residual disease (MRD) or donor chimerism assessment can be used in order to identify AML patients in morphological complete remission (CR) at high risk of impending relapse, who therefore may benefit from pre-DLI administration.
AML patients with recurrent genetic abnormalities such as NPM1 mutation, Core Binding Factor AML or acute promyelocitic leukemia (APL) are the best candidates for such an approach, given the availability of highly sensitive MRD markers; several studies have shown that the administration of pre-DLI improves disease outcomes [
31,
32].
However, since most AML patients lack an AML-specific MRD marker, multicolor flow cytometry (MFC) MRD is a good strategy to predict relapse and to guide a pre-emptive intervention [
33,
34].
4. Immune Checkpoint Inhibitors (ICI) as Immunotherapeutic Strategies for AML
Immune Checkpoints (IC) are proteins physiologically expressed on immune effector cells, acting as key regulators of T- and NK-mediated responses promoting self-tolerance. The discovery that cancer cells can mimic the IC ligands as a mechanism of immune escape led to the development of novel antitumor strategies with IC inhibitors (ICIs), which have become fundamental for the treatment of several solid organ malignancies [
42]. The only approved indication for treatment with ICIs of hematologic malignancies is R/R Hodgkin’s Lymphoma [
43].
In the AML setting, several ICIs have been investigated. The first ICI tested in AML was the CTLA-4 inhibitor Ipilimumab. It showed promising results in terms of the infiltration and expansion of effector T cells and reduced the activation of regulatory T cells as a single agent and in combination with Decitabine [
44].
Other strategies investigated in AML include treatments blocking the PD-1/PD-L1 axis.
A phase 2 study showed that the association between the PD1 inhibitor nivolumab and AZA determines an encouraging response rate and overall survival in patients with R/R AML; the greatest results were seen in the subset of patients who had not been previously exposed to hypomethylating agents [
45].
The association of the anti-PD-L1 human monoclonal antibody (mAb) Durvalumab with AZA led to unsatisfactory results with superimposable efficacy to AZA alone.
Monalizumab, a humanized immunoglobulin G4 (IgG4) mAb targeting the NK and T cell CI NKG2A, showed promising activity in AML murine models [
47,
48,
49,
50].
Sabatolimab is a humanized IgG4 mAb directed against the human T cell immunoglobulin domain and mucin domain-3 (TIM-3), an immune-regulatory receptor that is expressed on immune cells and leukemic blasts but not on hematopoietic stem cells.
5. Target Antigens for Cell-Based Immunotherapy in AML
The efficacy of immune-based therapies relies on the identification of target molecules, which allow for the recognition and killing of blast cells while sparing healthy hematopoietic precursors or other tissues. Therefore, to maximize efficacy and reduce off-target toxicities, an ideal target should be strongly expressed on the surface of AML blasts with no or limited expression on healthy bone marrow or extra hematopoietic cells [
52].
Unfortunately, leukemia-specific antigens consisting of tumor-specific proteins (i.e., resulting from gene mutations as mutated NPM1, FLT3-ITD and PML RARa), which would represent the ideal target for immunotherapy, are mainly expressed at the intracellular level and, therefore, are not suitable for immunotherapy strategies.
Several other antigens have already been explored and proposed as target for clinical use. Table 1 provides an overview of the most relevant target antigens for which the development of cell-based immunotherapy strategies is more advanced.
Table 1. Overview of the most relevant target antigens for which the development of cell-based immunotherapy strategies is more advanced.
| Target Antigen |
Type—Function |
% of Expression |
Potentialtarget Therapies |
| CD33 |
Lineage-specific
Sialic acid-binding receptor—role as negative regulator of cell activation |
Up to 90% |
CAR-T, CAR-NK, BiTEs, BiKEs, TRiKEs, DARPin |
| CD123 |
Lineage-specific
Alpha chain of the interleukin 3 receptor |
Variable
Higher in NPM1-mutated and FLT3-ITD AML |
CAR-T, CAR-NK, DARTs, BiTEs, ANKET, DARPin |
| CLL-1 |
Leukemia-associated
C-type lectin-like receptor—role in regulation of innate and adaptive immunity |
90–95% |
CAR-T, CAR-NK, BiTEs, TRiKEs, |
| (FLT3/CD135) |
Leukemia-associated
Class III receptor tyrosine kinases |
>90% |
CAR-T, BiTEs |
| NKG2DL |
Not tumor-specific
Ligand of C-type lectin-like receptor expressed on immune-effector cells-stimulates the cytotoxic activity |
Upregulated in neoplastic cells |
CAR-T, CAR-NK |
6. Chimeric Antigen Receptor Cells
6.1. CAR-T
Since the 1980s, T cells modified with the lentiviral chimeric antigen receptor (CAR) gene have been developed. The chimeric antigen receptor is made of a monoclonal antibody capable of binding to specifically identified target antigens, coupled with the activation of the intracellular proliferation signal domain [
79]. When the chimeric receptor binds to the antigen expressed in malignant cells, it stimulates the activation signal, leading to CAR-T cell activation and killing effects (
Figure 1B). When designing a CAR-T cell, the choice antigen receptor is very important as the CAR target should be highly expressed on tumor cells and weak or absent on healthy cells. CAR-T cells against the CD19 protein have been shown to be effective against acute lymphoblastic leukemia, B cell lymphoma and lymphocytic leukemia, and have been approved by the FDA and EMA in those settings.
In the AML setting, designing a CAR-T is particularly challenging, as potential targets are often expressed on healthy myeloid cell compartments, resulting in unacceptable toxicity and myelosuppression. Despite the limitation of the potential usefulness in AML, many CAR-T cell adoptive cell transfer therapies have been tested in early phase clinical trials [
80,
81].
6.2. CAR-NK
Given the potent anti-leukemic activity of NK cells and thanks to a better understanding of NK receptor functioning, a deep interest in exploiting anti-leukemic NK activity outside of the context of allogeneic HSCT has grown [
82,
83,
84,
85].
A pioneering study from Miller et al. showed that up to 1.5 × 107/haploidentical NK cells/Kg could be safely infused in AML and cancer patients following Fludarabine/Cyclophosphamide (Flu/Cy) immunosuppressive light conditioning with evidence of clinical responses without GvHD [
82].
Curti et al. reported on the feasibility and efficacy of adoptive immunotherapy with purified NK cells from a KIR-ligand mismatched haplo-identical donor in AML patients with R/R disease [
85]. NK selection was performed using a two-step immunomagnetic separation for the purification of CD56+CD3- cells from the donor’s leukapheresis. NK cell infusion was performed after Flu/Cy immunosuppressive therapy and was followed by the subcutaneous administration of Interleukin-2. No specific NK-cell-related toxicities were observed. Donor-versus-recipient alloreactive NK cells were demonstrated in vivo by the detection of donor-derived NK clones and adoptively transferred NK cells were alloreactive against the recipient’s leukemic cells.
Albeit promising, these approaches have the limitation of requiring a haploidentical donor of NK cells. In order to overcome this issue, more recently, chimeric antigen receptor-modified NK cell therapy (CAR-NK) has been developed as a novel therapeutic option for hematological malignancies [
87]. NK cells engineered with CAR expression maintain their capability of being stimulated by innate receptors, whilst their antigen recognition is redirected towards CAR-specific targets (
Figure 1B).
7. Strategies for T and NK Cells Engagement
7.1. BiTEs and DARTs
The term “bispecific antibodies” refers to a family of molecules specifically designed to simultaneously bind two different antigens located on immune effector cells and tumor cells. The idea of a hybrid antibody capable of activating effector T cells against target antigens dates back to 1985, and the CD3 polypeptides on T cells were identified to be the T cell engager component of the bispecific antibody [
90,
91].
Based on these data, the first research in this field focused on the creation of a bispecific antibody able to involve the cytotoxic activity of T cells with the development of molecular constructs, allowing the simultaneous binding of target antigens on the surface of malignant cells and the CD3 component of the T cell receptor complex (TCR) [
92,
93].
In the setting of hematological malignancies, the first bispecific T cell engager (BiTE) produced was blinatumomab, directed against CD3 on T cells and CD19 on blast cells, currently approved for the treatment of patients with relapsed/refractory B cell-precursor acute lymphoblastic leukemia (B-ALL
The great success achieved by blinatumomab set the basis for further exploration of bispecific antibodies in other hematological malignancies, such as AML, by targeting myeloid-specific antigens such as CD33, CD123 and CLL-1.
7.2. BiKEs and TRiKEs
A further evolution of immunotherapy is based on bispecific antibodies capable of engaging with NK cells. These include the development of Bi- and Tri-specific killer cell engagers, also known as BiKEs and TriKEs, which produce more efficient immunological synapses between NK cells and tumor cells.
These molecules share with BiTEs the backbone structure consisting of at least two single chain variable fragments (scFVs) connected by a linker sequence. The main difference consists of the engagement and activation of NK cells through the binding of CD16 by the scFV, while the other scFV remains specific to the tumor-associated target. In addition, TRiKEs, in their structure, present an additional portion consisting of an IL-15 crosslinker, which is able to expand the NK response. Anti-CD16 scFV BiKEs and TriKEs can further activate NK cells via CD16-Fc interaction by mediating the ADCC of opsonized target cells in view of the CD16 polymorphism and the presence of low-affinity CD16 allotypes. When compared to the use of mAbs, BiKEs and TriKEs show several advantages, such as increased biodistribution given their smaller composition, greater flexibility and indeed lower immunogenicity (
Figure 1C).
7.3. DARPins
Most recent strategies exploit the simultaneous targeting of multiple TAAs, in order to overcome immunotherapy limitations due to the heterogeneous expression of TAAs on AML blasts. In this way, DARPins (designed ankyrin repeat proteins) represent a novel class of small, single-domain, non-immunoglobulin proteins with multiple therapeutic potentials (
Figure 1C). MP0533 is the first half-life extended avidity-engineered CD3 engaging DARPin able to simultaneously target CD33, CD123 and CD70. Preclinical studies have demonstrated that MP0533 is effective and able to induce T cell activation and the killing of AML blasts, with a better selectivity towards LSC over healthy hematopoietic stem cells (HSCs).
7.4. ANKETsTM
ANKETs™ (Antibody-based NK cell Engagers) are multi-specific natural killer (NK) cell engagers that target specific TAAs on neoplastic cells (Figure 1C).
Preclinical studies have shown that the trifunctional NKp46-CD16a-NK engager (NKCE) targeting CD123 has good control of AML cell proliferation. Through its bond to NKp46, CD123-NKCE specifically targets NK cells and has potent antitumor activity against primary AML blasts, inducing NK cell activation and cytokine secretion. Mo
8. New Toxicity Profile of Cell-Based Immunotherapy
The aim of cancer immunotherapy is to eradicate malignant cells by harnessing the power of the immune system. In this scenario, clinicians must face new specific therapy-related toxicities induced by the activation of immune-effectors. The most well-addressed side effect of novel immunological therapies is represented by cytokine release syndrome (CRS), consisting of a non-specific and abnormal activation of the immune system with the release of cytokines such as TNFα, IL-6 and IFNγ, and further activation of lymphocytes and myeloid cells.
The clinical manifestations and severity of immune system activation and cytokine release are variable from constitutional symptoms, including fever, malaise, myalgia, nausea, vomiting and headache to potentially life-threatening end-organ damage, including cardiovascular dysfunction, adult respiratory distress syndrome (ARDS), renal/hepatic failure and neurological manifestations.
The management of low-grade CRS consists of general supportive care measures, while grade ≥ 3 CRS generally requires immunosuppressive therapies, such as steroids and the anti-IL6 antibody tocilizumab. Vasopressors are required in case of hemodynamic instability unresponsive to fluid repletion [
107].
Immune-effector cell-associated neurological syndrome (ICANS) is another possible toxicity of immunotherapy mostly associated with CAR-T infusion. Its clinical manifestations consist of progressive mental status changes, varying from mild symptoms such as confusion or disorientation to more severe cognitive impairment with agraphia and aphasia. ICANS can also occur in concomitance with CRS [
108]. Its treatment mainly consists of steroids, in particular high doses of dexamethasone, eventually associated with tocilizumab in the case of CRS coexistence.
These emerging toxicities require close monitoring and intense support, especially in the cases of CRS or ICANS, requiring the prompt administration of immunosuppressive drugs and ICU transfer in critically ill patients.
This entry is adapted from the peer-reviewed paper 10.3390/jcm12185824