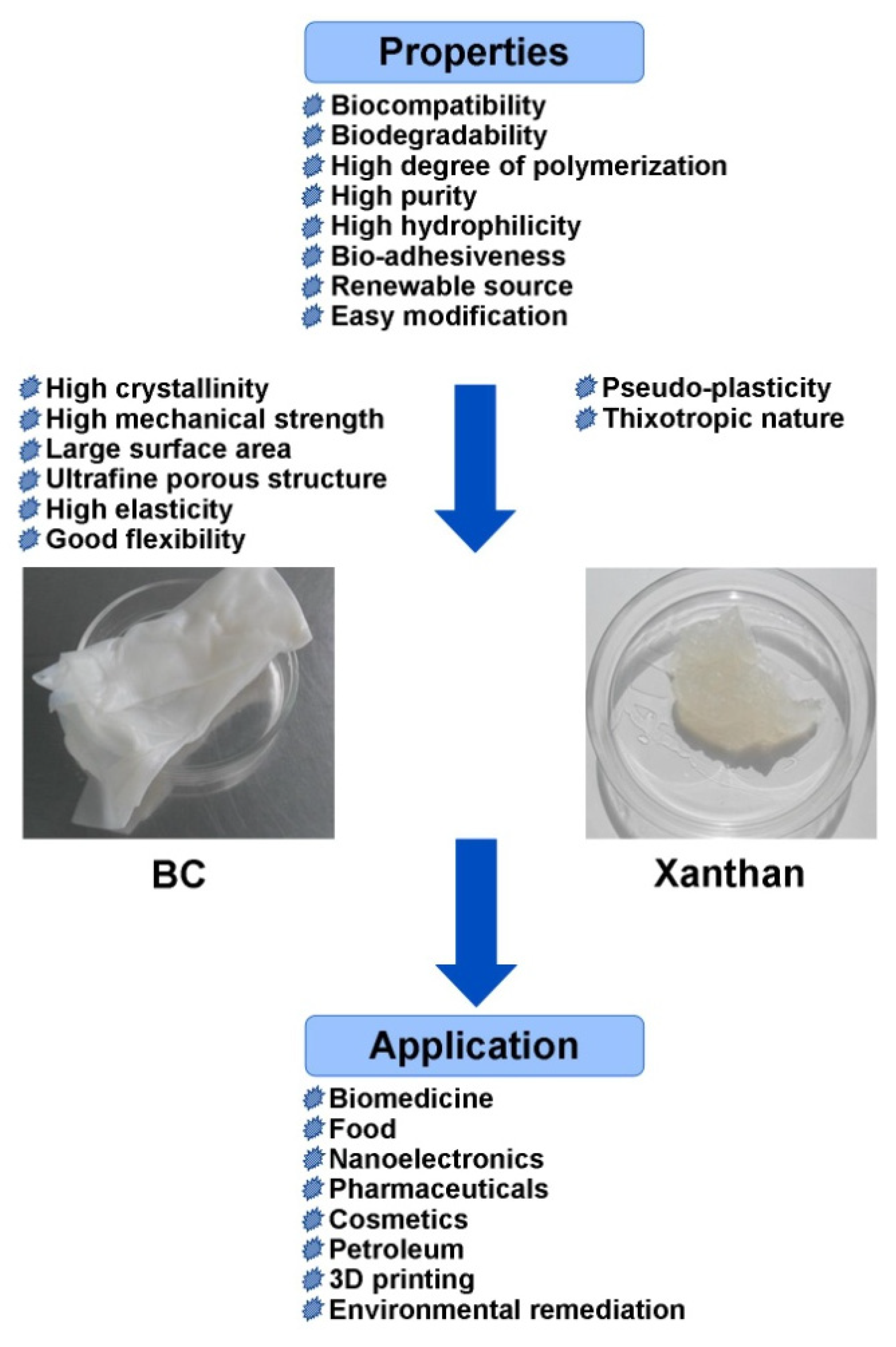
Degradable biopolymers have become increasingly important as potential environmentally friendly biomaterials, providing a wide range of applications in various fields. Bacterial exopolysaccharides (EPSs) are biomacromolecules, which due to their unique properties have found applications in biomedicine, foodstuff, textiles, cosmetics, petroleum, pharmaceuticals, nanoelectronics, and environmental remediation. One of the important commercial polysaccharides produced on an industrial scale is xanthan. In recent years, the range of its application has expanded significantly. Bacterial cellulose (BC) is another unique EPS with a rapidly increasing range of applications.
- biopolymers
- biomacromolecules
- bacterial exopolysaccharides
- xanthan
- bacterial cellulose
1. Properties and Biosynthesis of Bacterial Cellulose and Xanthan
2. Applications of Bacterial Cellulose and Xanthan
This entry is adapted from the peer-reviewed paper 10.3390/ijms241914608
References
- Chang, I.; Lee, M.; Tran, A.T.P.; Lee, S.; Kwon, Y.M.; Im, J.; Cho, G.C. Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transp. Geotech. 2020, 24, 100385.
- Furtado, I.F.S.P.C.; Sydney, E.B.; Rodrigues, S.A.; Sydney, A.C.N. Xanthan gum: Applications, challenges, and advantages of this asset of biotechnological origin. Biotechnol. Res. Innov. 2022, 6, e202204.
- Berninger, T.; Dietz, N.; González López, Ó. Water-soluble polymers in agriculture: Xanthan gum as eco-friendly alternative to synthetics. Microb. Biotechnol. 2021, 14, 1881–1896.
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical Applications of Bacterial Exopolysaccharides: A Review. Polymers 2021, 13, 530.
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of bacterial cellulose from alternative cheap and waste resources: A step for cost reduction with positive environmental aspects. Korean J. Chem. Eng. 2020, 37, 925–937.
- Hsieh, Y.C.; Yano, H.; Nogi, M.; Eichhorn, S.J. An estimation of the young’s modulus of bacterial cellulose filaments. Cellulose 2008, 15, 507–513.
- Choi, C.N.; Song, H.J.; Kim, M.J.; Chang, M.H.; Kim, S.J. Properties of bacterial cellulose produced in a pilot-scale spherical type bubble column bioreactor. Korean J. Chem. Eng. 2009, 26, 136–140.
- Jadczak, K.; Ochędzan-Siodłak, W. Bacterial cellulose: Biopolymer with novel medical applications. J. Biomater. Appl. 2023, 38, 51–63.
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.D.S.; Castro, G.R. Bacterial Cellulose-Based Materials as Dressings for Wound Healing. Pharmaceutics 2023, 15, 424.
- Manan, S.; Wajid Ullah, M.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972.
- Popa, L.; Ghica, M.V.; Tudoroiu, E.-E.; Ionescu, D.G.; Dinu-Pîrvu, C.E. Bacterial Cellulose—A Remarkable Polymer as a Source for Biomaterials Tailoring. Materials 2022, 15, 1054.
- Navya, P.V.; Gayathri, V.; Samanta, D.; Sampath, S. Macromolecules Bacterial Cellulose: A Promising Biopolymer with Interesting Properties and Applications. Int. J. Biol. Macromol. 2022, 220, 435–461.
- Choi, S.M.; Shin, E.J. The Nanofication and Functionalization of Bacterial Cellulose and Its Applications. Nanomaterials 2020, 10, 406.
- Ullah, M.W.; Rojas, O.J.; McCarthy, R.R.; Yang, G. Editorial: Nanocellulose: A Multipurpose Advanced Functional Material. Front. Bioeng. Biotechnol. 2021, 9, 738779.
- Ul-Islam, M.; Ahmad, F.; Fatima, A.; Shah, N.; Yasir, S.; Ahmad, M.W.; Manan, S.; Ullah, M.W. Ex situ Synthesis and Characterization of High Strength Multipurpose Bacterial Cellulose- Aloe vera Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 601988.
- Wu, M.; Qu, J.; Shen, Y.; Dai, X.; Wei, W.; Shi, Z.; Li, G.; Ma, T. Gel properties of xanthan containing a single repeating unit with saturated pyruvate produced by an engineered Xanthomonas campestris CGMCC 15155. Food Hydrocoll. 2019, 87, 747–757.
- Fitzpatrick, P.; Meadows, J.; Ratcliffe, I.; Williams, P.A. Control of the properties of xanthan/glucomannan mixed gels by varying xanthan fine structure. Carbohydr. Polym. 2013, 92, 1018–1025.
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604.
- Moffat, J.; Morris, V.J.; Al-Assaf, S.; Gunning, A.P. Visualisation of xanthan conformation by atomic force microscopy. Carbohydr. Polym. 2016, 148, 380–389.
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Masoodi, F. Advances in xanthan gum production, modifications and its applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328.
- Al-Muhanna, M.K.; Anwar, N.; Hasnain, S.; Nayak, A.K. Chapter 1–Synthesis of tailor-made polysaccharides: An overview. In Tailor-Made Polysaccharides in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–27.
- Şen, E.; Demirci, A.S.; Palabiyik, I. Xanthan gum characterization and production kinetics from pomace of Vitis vinifera. J. Food Process. Preserv. 2022, 46, e17098.
- Maurya, D.K.; Kumar, A.; Chaurasiya, U.; Hussain, T.; Singh, S.K. Modern era of microbial biotechnology: Opportunities and future prospects. Microbiomes Plant Health Panoply Appl. 2020, 1, 317–343.
- Pirsa, S.; Hafezi, K. Hydrocolloids: Structure, preparation method, and application in food industry. Food Chem. 2023, 399, 133967.
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides Producing Bacteria: A Review. Microorganisms 2023, 11, 1541.
- Tang, J.L.; Tang, D.J.; Dubrow, Z.E.; Bogdanove, A.; An, S.Q. Xanthomonas campestris Pathovars. Trends Microbiol. 2021, 29, 182–183.
- Gumus, T.; Sukru Demirci, A.; Mirik, M.; Arici, M.; Aysan, Y. Xanthan gum production of Xanthomonas spp. isolated from different plants. Food Sci. Biotechnol. 2010, 19, 201–206.
- Niknezhad, S.V.; Asadollahi, M.A.; Zamani, A.; Biria, D. Production of xanthan gum by free and immobilized cells of Xanthomonas campestris and Xanthomonas pelargonii. Int. J. Biol. Macromol. 2016, 82, 751–756.
- Ramezani, A.; Jafari, M.; Goodarzi, T.; Alavi, S.M.; Salmanian, A.H.; Azin, M. Lactose consuming strains of Xanthomonas citri subsp. citri (Xcc) insight into the emergence of natural field resources for xanthan gum production. World J. Microbiol. Biotechnol. 2014, 30, 1511–1517.
- Revin, V.V.; Liyas’kina, E.V.; Sapunova, N.B.; Bogatyreva, A.O. Isolation and characterization of the strains producing bacterial cellulose. Microbiology 2020, 14, 86–95.
- Kaczmarek, M.; Jedrzejczak-Krzepkowska, M.; Ludwicka, K. Comparative Analysis of Bacterial Cellulose Membranes Synthesized by Chosen Komagataeibacter Strains and Their Application Potential. Int. J. Mol. Sci. 2022, 23, 3391.
- Lee, Y.S.; Kim, J.Y.; Cha, M.Y.; Kang, H.C. Characteristics of biocellulose by Gluconobacter uchimurae GYS15. J. Soc. Cosmet. Sci. Korea 2016, 42, 247–255.
- Nie, W.; Zheng, X.; Feng, W.; Liu, Y.; Li, Y.; Liang, X. Characterization of Bacterial Cellulose Produced by Acetobacter Pasteurianus MGC-N8819 Utilizing Lotus Rhizome. LWT 2022, 165, 113763.
- Hasanin, M.S.; Abdelraof, M.; Hashem, A.H.; El Saied, H. Sustainable bacterial cellulose production by Achromobacter using mango peel waste. Microb. Cell Fact. 2023, 22, 24.
- Barnhart, D.M.; Su, S.; Farrand, S.K. A signaling pathway involving the diguanylate cyclase CelR and the response regulator DivK controls cellulose synthesis in Agrobacterium tumefaciens. J. Bacteriol. 2014, 196, 1257–1274.
- Gao, G.; Ji, K.; Zhang, Y.; Liu, X.; Dai, X.; Zhi, B.; Cao, Y.; Liu, D.; Wu, M.; Li, G.; et al. Microbial enhanced oil recovery through deep profile control using a conditional bacterial cellulose-producing strain derived from Enterobacter sp. FY-07. Microb. Cell. Fact. 2020, 19, 59.
- Ardré, M.; Dufour, D.; Rainey, P.B. Causes and Biophysical Consequences of Cellulose Production by Pseudomonas fluorescens SBW25 at the Air-Liquid Interface. J. Bacteriol. 2019, 201, e00110-19.
- Pérez-Mendoza, D.; Romero-Jiménez, L.; Rodríguez-Carvajal, M.Á.; Lorite, M.J.; Muñoz, S.; Olmedilla, A.; Sanjuán, J. The Role of Two Linear β-Glucans Activated by c-di-GMP in Rhizobium etli CFN42. Biology 2022, 11, 1364.
- Fratty, I.S.; Shachar, D.; Katsman, M.; Yaron, S. The activity of BcsZ of Salmonella Typhimurium and its role in Salmonella-plants interactions. Front. Cell. Infect. Microbiol. 2022, 12, 967796.
- Bagewadi, Z.K.; Bhavikatti, J.S.; Muddapur, U.M.; Yaraguppi, D.A.; Mulla, S.I. Statistical Optimization and Characterization of Bacterial Cellulose Produced by Isolated Thermophilic Bacillus licheniformis Strain ZBT2. Carbohydr. Res. 2020, 491, 107979.
- Li, G.; Wang, L.; Deng, Y.; Wei, Q. Research progress of the biosynthetic strains and pathways of bacterial cellulose. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab071.
- McNamara, J.T.; Morgan, J.L.; Zimmer, J. A molecular description of cellulose biosynthesis. Annu. Rev. Biochem. 2015, 84, 895–921.
- Ryngajłło, M.; Jędrzejczak-Krzepkowska, M.; Kubiak, K.; Ludwicka, K.; Bielecki, S. Towards control of cellulose biosynthesis by Komagataeibacter using systems-level and strain engineering strategies: Current progress and perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 6565–6585.
- Ross, P.; Weinhouse, H.; Aloni, Y.; Michaeli, D.; Weinberger-Ohana, P.; Mayer, R.; Braun, S.; de Vroom, E.; van der Marel, G.A.; van Boom, J.H.; et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 1987, 325, 279–281.
- Buldum, G.; Mantalaris, A. Systematic Understanding of Recent Developments in Bacterial Cellulose Biosynthesis at Genetic, Bioprocess and Product Levels. Int. J. Mol. Sci. 2021, 22, 7192.
- Bimmer, M.; Mientus, M.; Klingl, A.; Ehrenreich, A.; Liebl, W. The Roles of the Various Cellulose Biosynthesis Operons in Komagataeibacter hansenii ATCC 23769. Appl. Environ. Microb. 2022, 88, e02460-21.
- Kondo, T.; Nakamura, Y.; Nojima, S.; Yao, M.; Imai, T. The BcsD subunit of type I bacterial cellulose synthase interacts dynamically with the BcsAB catalytic core complex. FEBS Lett. 2022, 596, 3069–3086.
- Balíková, K.; Farkas, B.; Matúš, P.; Urík, M. Prospects of Biogenic Xanthan and Gellan in Removal of Heavy Metals from Contaminated Waters. Polymers 2022, 14, 5326.
- Choi, S.; Rao, K.; Zo, S.; Shin, E.; Han, S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080.
- Wahid, F.; Huang, L.-H.; Zhao, X.-Q.; Li, W.-C.; Wang, Y.-Y.; Jia, S.-R.; Zhong, C. Bacterial cellulose and its potential for biomedical applications. Biotechnol. Adv. 2021, 53, 107856.
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent advances and applications of bacterial cellulose in biomedicine. Polymers 2021, 13, 412.
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced Functional Materials Based on Nanocellulose for Pharmaceutical/Medical Applications. Pharmaceutics 2021, 13, 1125.
- Pandit, A.; Kumar, R. A Review on Production, Characterization and Application of Bacterial Cellulose and Its Biocomposites. J. Polym. Environ. 2021, 29, 2738–2755.
- Qian, H.; Liu, J.; Wang, X.; Pei, W.; Fu, C.; Ma, M.; Huang, C. The state-of-the-art application of functional bacterial cellulose-based materials in biomedical fields. Carbohydr. Polym. 2023, 300, 120252.
- Tang, K.Y.; Heng, J.Z.X.; Chai, C.H.T.; Chan, C.Y.; Low, B.Q.L.; Chong, S.M.E.; Loh, H.Y.; Li, Z.; Ye, E.; Loh, X.J. Modified Bacterial Cellulose for Biomedical Applications. Chem. Asian J. 2022, 17, 202200598.
- Habibi, H.; Khosravi-Darani, K. Effective variables on production and structure of xanthan gum and its food applications: A review. Biocatal. Agric. Biotechnol. 2017, 10, 130–140.
- Abu Elella, M.H.; Goda, E.S.; Gab-Allah, M.A.; Hong, S.E.; Pandit, B.; Lee, S.; Gamal, H.; Rehman, A.U.; Yoon, K.R. Xanthan gum-derived materials for applications in environment and eco-friendly materials: A review. J. Environ. Chem. Eng. 2020, 9, 104702.
- Patel, J.; Maji, B.; Moorthy, N.S.H.N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136.
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Use of xanthan gum for whole cell immobilization and its impact in bioremediation—A review. Bioresour. Technol. 2022, 351, 126918.
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 42035.
- Cubas, A.L.V.; Provin, A.P.; Dutra, A.R.A.; Mouro, C.; Gouveia, I.C. Advances in the Production of Biomaterials through Kombucha Using Food Waste: Concepts, Challenges, and Potential. Polymers 2023, 15, 1701.
- Kamal, T.; Ul-Islam, M.; Fatima, A.; Ullah, M.W.; Manan, S. Cost-Effective Synthesis of Bacterial Cellulose and Its Applications in the Food and Environmental Sectors. Gels 2022, 8, 552.
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546.
- Jiang, Z.; Ngai, T. Recent Advances in Chemically Modified Cellulose and Its Derivatives for Food Packaging Applications: A Review. Polymers 2022, 14, 1533.
- Singh, A.K.; Itkor, P.; Lee, Y.S. State-of-the-Art Insights and Potential Applications of Cellulose-Based Hydrogels in Food Packaging: Advances towards Sustainable Trends. Gels 2023, 9, 433.
- Atta, O.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, W.M.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419.
- Cielecka, I.; Szustak, M.; Kalinowska, H.; Gendaszewska-Darmach, E.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Glycerolplasticized bacterial nanocellulose-based composites with enhanced flexibility and liquid sorption capacity. Cellulose 2019, 26, 5409–5426.
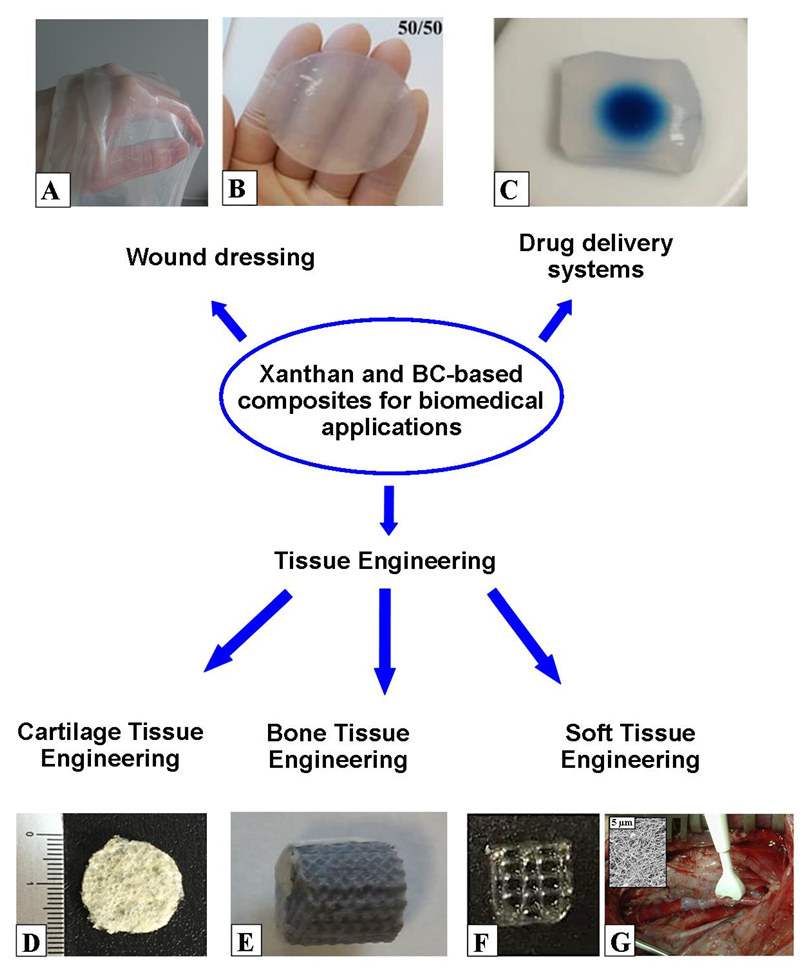
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610.
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106.
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571.
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest Advances on Bacterial Cellulose-Based Antibacterial Materials as Wound Dressings. Front. Bioeng. Biotechnol. 2020, 8, 593768.
- de Amorim, J.D.P.; da Silva Junior, C.J.G.; de Medeiros, A.D.M.; do Nascimento, H.A.; Sarubbo, M.; de Medeiros, T.P.M.; Costa, A.F.S.; Sarubbo, L.A. Bacterial Cellulose as a Versatile Biomaterial for Wound Dressing Application. Molecules 2022, 27, 5580.
- Meng, S.; Wu, H.; Xiao, D.; Lan, S.; Dong, A. Recent advances in bacterial cellulose-based antibacterial composites for infected wound therapy. Carbohydr. Polym. 2023, 316, 121082.
- Pasaribu, K.M.; Ilyas, S.; Tamrin, T.; Radecka, I.; Swingler, S.; Gupta, A.; Stamboulis, A.G.; Gea, S. Bioactive bacterial cellulose wound dressings for burns with collagen in-situ and chitosan ex-situ impregnation. Int. J. Biol. Macromol. 2023, 230, 123118.
- Tang, S.; Gong, Z.; Wang, Z.; Gao, X.; Zhang, X. Multifunctional hydrogels for wound dressings using xanthan gum and polyacrylamide. Int. J. Biol. Macromol. 2022, 217, 944–955.
- Singh, S.; Nwabor, O.F.; Sukri, D.M.; Wunnoo, S.; Dumjun, K.; Lethongkam, S.; Kusolphat, P.; Hemtanon, N.; Klinprathum, K.; Sunghan, J.; et al. Poly (vinyl alcohol) copolymerized with xanthan gum/hypromellose/sodium carboxymethyl cellulose dermal dressings functionalized with biogenic nanostructured materials for antibacterial and wound healing application. Int. J. Biol. Macromol. 2022, 216, 235–250.
- Gutierrez-Reyes, J.E.; Caldera-Villalobos, M.; Claudio-Rizo, J.A.; Cabrera-Munguía, D.A.; Becerra-Rodriguez, J.J.; Soriano-Corral, F.; Herrera-Guerrero, A. Smart collagen/xanthan gum-based hydrogels with antibacterial effect, drug release capacity and excellent performancein vitrobioactivity for wound healing application. Biomed. Mater. 2023, 18, 035011.
- Unalan, I.; Schruefer, S.; Schubert, D.W.; Boccaccini, A.R. 3D-Printed Multifunctional Hydrogels with Phytotherapeutic Properties: Development of Essential Oil-Incorporated ALG-XAN Hydrogels for Wound Healing Applications. ACS Biomater. Sci. Eng. 2023, 9, 4149–4167.
- Liang, Y.; Chitrakar, B.; Liu, Z.; Ming, X.; Xu, D.; Mo, H.; Shi, C.; Zhu, X.; Hu, L.; Li, H. Preparation and characterization of 3D-printed antibacterial hydrogel with benzyl isothiocyanate. Int. J. Bioprint. 2023, 9, 671.
- Alves, A.; Miguel, S.P.; Araujo, A.R.T.S.; de Jesús Valle, M.J.; Sánchez Navarro, A.; Correia, I.J.; Ribeiro, M.P.; Coutinho, P. Xanthan Gum–Konjac Glucomannan Blend Hydrogel for Wound Healing. Polymers 2020, 12, 99.
- Das, S.; Ghosh, B.; Sarkar, K. Nanocellulose as sustainable biomaterials for drug delivery. Sens. Int. 2022, 3, 100135.
- Mensah, A.; Chen, Y.; Christopher, N.; Wei, Q. Membrane Technological Pathways and Inherent Structure of Bacterial Cellulose Composites for Drug Delivery Bioengineering. Bioengineering 2022, 9, 3.
- Hasan, N.; Rahman, L.; Kim, S.-H.; Cao, J.; Arjuna, A.; Lallo, S.; Jhun, B.H.; Yoo, J.-W. Recent advances of nanocellulose in drug delivery systems. J. Pharm. Investig. 2020, 50, 553–572.
- Qiu, A.; Wang, Y.; Zhang, G.; Wang, H. Natural Polysaccharide-Based Nanodrug Delivery Systems for Treatment of Diabetes. Polymers 2022, 14, 3217.
- Huo, Y.; Liu, Y.; Xia, M.; Du, H.; Lin, Z.; Li, B.; Liu, H. Nanocellulose-Based Composite Materials Used in Drug Delivery Systems. Polymers 2022, 14, 2648.
- Lunardi, V.B.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Yuliana, M.; Sunarso, J.; Ju, Y.-H.; Ismadji, S. Nanocelluloses: Sources, Pretreatment, Isolations, Modification, and Its Application as the Drug Carriers. Polymers 2021, 13, 2052.
- Chung, C.K.; Beekmann, U.; Kralisch, D.; Bierau, K.; Chan, A.; Ossendorp, F.; Cruz, L.J. Bacterial Cellulose as Drug Delivery System for Optimizing Release of Immune Checkpoint Blocking Antibodies. Pharmaceutics 2022, 14, 1351.
- Jadav, M.; Pooja, D.; Adams, D.J.; Kulhari, H. Advances in Xanthan Gum-Based Systems for the Delivery of Therapeutic Agents. Pharmaceutics 2023, 15, 402.
- Kala, S.; Gurudiwan, P.; Juyal, D. Formulation and Evaluation of Besifloxacin Loaded In Situ Gel For Ophthalmic Delivery. Pharm. Biosci. J. 2018, 6, 36–40.
- Garg, R.; Kumar, V.; Sharma, V. Design and Characterization of Flucytosine Loaded Bioadhesive In Situ Ophthalmic Gel for Improved Bioavailability. Pharm. Biosci. J. 2019, 7, 17–20.
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/Xanthan Gum Based Hydrogels as Potential Carrier for an Antiviral Drug: Fabrication, Characterization, and Safety Evaluation. Front. Chem. 2020, 8, 50.
- Ribeiro, M.; Boudoukhani, M.; Belmonte-Reche, E.; Genicio, N.; Sillankorva, S.; Gallo, J.; Rodríguez-Abreu, C.; Moulai-Mostefa, N.; Bañobre-López, M. Xanthan-Fe3O4 Nanoparticle Composite Hydrogels for Non-Invasive Magnetic Resonance Imaging and Magnetically Assisted Drug Delivery. ACS Appl. Nano Mater. 2021, 4, 7712–7729.
- Inoue, B.S.; Streit, S.; Schneider, A.L.D.S.; Meier, M.M. Bioactive bacterial cellulose membrane with prolonged release of chlorhexidine for dental medical application. Int. J. Biol. Macromol. 2020, 148, 1098–1108.
- Ul, S.; Ul-islam, M.; Ahsan, H.; Bilal, M.; Shehzad, A.; Fatima, A.; Kyung, J.; Sup, Y. Potential applications of bacterial cellulose and its composites for cancer treatment. Int. J. Biol. Macromol. 2020, 168, 301–309.
- Cacicedo, M.L.; Islan, G.A.; León, I.E.; Álvarez, V.A.; Chourpa, I.; Allard-Vannier, E.; Castro, G.R. Bacterial cellulose hydrogel loaded with lipid nanoparticles for localized cancer treatment. Colloids Surf. B Biointerfaces 2018, 170, 596–608.
- Zhang, L.K.; Du, S.; Wang, X.; Jiao, Y.; Yin, L.; Zhang, Y.; Guan, Y.Q. Bacterial cellulose based composites enhanced transdermal drug targeting for breast cancer treatment. Chem. Eng. J. 2019, 370, 749–759.
- Zhou, J.-J.; Li, X.-H.; He, P.-Y.; Qi, F.-Y.; Ullah, M.W.; Li, S.-J.; Liu, Y.-T.; Bu, L.-L.; Yang, G.; Sun, Z.-J. Implantable versatile oxidized bacterial cellulose membrane for postoperative HNSCC treatment via photothermal-boosted immunotherapy. Nano Res. 2023, 16, 951–963.
- Trombino, S.; Serini, S.; Cassano, R.; Calviello, G. Xanthan gum-based materials for omega-3 PUFA delivery: Preparation, characterization and antineoplastic activity evaluation. Carbohydr. Polym. 2019, 208, 431–440.
- Webster, T.J.; Singh, S.; Kotla, N.G.; Tomar, S.; Maddiboyina, B.; Sharma, D.; Sunnapu, O. A nanomedicine-promising approach to provide an appropriate colon-targeted drug delivery system for 5-fluorouracil. Int. J. Nanomed. 2015, 10, 7175–7182.
- Anwar, M.; Pervaiz, F.; Shoukat, H.; Noreen, S.; Shabbir, K.; Majeed, A.; Ijaz, S. Formulation and evaluation of interpenetrating network of xanthan gum and polyvinylpyrrolidone as a hydrophilic matrix for controlled drug delivery system. Polym. Bull. 2021, 78, 59–80.
- Feng, Z.; Xu, J.; Ni, C. Preparation of redox responsive modified xanthan gum nanoparticles and the drug controlled release. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 994–1001.
- Zhang, L.; Xu, J.; Wen, Q.; Ni, C. Preparation of xanthan gum nanogels and their pH/redox responsiveness in controlled release. J. Appl. Polym. Sci. 2019, 136, 6–11.
- Anghel, N.; Apostol, I.; Dinu, M.V.; Dimitriu, C.D.; Spiridon, I.; Verestiuc, L. Xanthan-Based Materials as a Platform for Heparin Delivery. Molecules 2023, 28, 2757.
- Gorgieva, S. Bacterial Cellulose as a Versatile Platform for Research and Development of Biomedical Materials. Processes 2020, 8, 624.
- da Silva, I.G.R.; dos Santos Pantoja, B.T.; Almeida, G.H.D.R.; Carreira, A.C.O.; Miglino, M.A. Bacterial Cellulose and ECM Hydrogels: An Innovative Approach for Cardiovascular Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 3955.
- Raut, M.P.; Asare, E.; Syed Mohamed, S.M.D.; Amadi, E.N.; Roy, I. Bacterial Cellulose-Based Blends and Composites: Versatile Biomaterials for Tissue Engineering Applications. Int. J. Mol. Sci. 2023, 24, 986.
- Andersson, J.; Stenhamre, H.; Bäckdahl, H.; Gatenholm, P. Behavior of human chondrocytes in engineered porous bacterial cellulose scaffolds. J. Biomed. Mater. Res. A 2010, 94, 1124–1132.
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431.
- Yin, N.; Stilwell, M.D.; Santos, T.M.; Wang, H.; Weibel, D.B. Agarose particle-templated porous bacterial cellulose and its application in cartilage growth in vitro. Acta Biomater. 2015, 12, 129–138.
- Wu, J.; Yin, N.; Chen, S.; Weibel, D.B.; Wang, H. Simultaneous 3D cell distribution and bioactivity enhancement of bacterial cellulose (BC) scaffold for articular cartilage tissue engineering. Cellulose 2019, 26, 2513–2528.
- Xun, X.; Li, Y.; Zhu, X.; Zhang, Q.; Lu, Y.; Yang, Z.; Wan, Y.; Yao, F.; Deng, X.; Luo, H. Fabrication of Robust, Shape Recoverable, Macroporous Bacterial Cellulose Scaffolds for Cartilage Tissue Engineering. Macromol. Biosci. 2021, 2021, 2100167.
- Gu, L.; Li, T.; Song, X.; Yang, X.; Li, S.; Chen, L.; Liu, P.; Gong, X.; Chen, C.; Sun, L. Preparation and characterization of methacrylated gelatin/bacterial cellulose composite hydrogels for cartilage tissue engineering. Regen. Biomater. 2020, 7, 195–202.
- Horbert, V.; Boettcher, J.; Foehr, P.; Kramer, F.; Udhardt, U.; Bungartz, M.; Brinkmann, O.; Burgkart, R.H.; Klemm, D.O.; Kinne, R.W. Laser perforation and cell seeding improve bacterial nanocellulose as a potential cartilage implant in the in vitro cartilage punch model. Cellulose 2019, 26, 647–664.
- Yang, J.; Wang, L.; Zhang, W.; Sun, Z.; Li, Y.; Yang, M.; Zeng, D.; Peng, B.; Zheng, W.; Jiang, X.; et al. Reverse reconstruction and bioprinting of bacterial cellulose-based functional total intervertebral disc for therapeutic implantation. Small 2018, 14, 1702582.
- Tazi, N.; Zhang, Z.; Messaddeq, Y.; Almeida-Lopes, L.; Zanardi, L.M.; Levinson, D.; Rouabhia, M. Hydroxyapatite bioactivated bacterial cellulose promotes osteoblast growth and the formation of bone nodules. AMB Express 2012, 2, 61.
- Dubey, S.; Mishra, R.; Roy, P.; Singh, R.P. 3-D macro/microporous-nanofibrous bacterial cellulose scaffolds seeded with BMP-2 preconditioned mesenchymal stem cells exhibit remarkable potential for bone tissue engineering. Int. J. Biol. Macromol. 2021, 167, 934–946.
- Gutiérrez-Hernández, J.M.; Escobar-García, D.M.; Escalante, A.; Flores, H.; González, F.J.; Gatenholm, P.; Toriz, G. In vitro evaluation of osteoblastic cells on bacterial cellulose modified with multi-walled carbon nanotubes as scaffold for bone regeneration. Mater. Sci. Eng. C 2017, 75, 445–453.
- Klinthoopthamrong, N.; Chaikiawkeaw, D.; Phoolcharoen, W.; Rattanapisit, K.; Kaewpungsup, P.; Pavasant, P.; Hoven, V.P. Bacterial cellulose membrane conjugated with plant-derived osteopontin: Preparation and its potential for bone tissue regeneration. Int. J. Biol. Macromol. 2020, 149, 51–59.
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020, 122, 109365.
- Zhang, C.; Cao, J.; Zhao, S.; Luo, H.; Yang, Z.; Gama, M.; Zhang, Q.; Su, D.; Wan, Y. Biocompatibility evaluation of bacterial cellulose as a scaffold material for tissue-engineered corneal stroma. Cellulose 2020, 27, 2775–2784.
- Wippermann, J.; Schumann, D.; Klemm, D.; Kosmehl, H.; Salehi-Gelani, S.; Wahlers, T. Preliminary Results of Small Arterial Substitute Performed with a New Cylindrical Biomaterial Composed of Bacterial Cellulose. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 592–596.
- Pértile, R.; Moreira, S.; Andrade, F.; Domingues, L.; Gama, M. Bacterial cellulose modified using recombinant proteins to improve neuronal and mesenchymal cell adhesion. Biotechnol. Prog. 2012, 28, 526–532.
- Kim, D.Y.; Park, S.E.; Jo, I.S.; Kim, S.M.; Kang, D.H.; Cho, S.P.; Park, J.B.; Hong, B.H.; Yoon, M.H. Multiscale modulation of nanocrystalline cellulose hydrogel via nanocarbon hybridization for 3D neuronal bilayer formation. Small 2017, 13, 1700331.
- Hosseini, H.; Zirakjou, A.; Goodarzi, V.; Mousavi, S.M.; Khonakdar, H.A.; Zamanlui, S. Lightweight aerogels based on bacterial cellulose/silver nanoparticles/ polyaniline with tuning morphology of polyaniline and application in soft tissue engineering. Int. J. Biol. Macromol. 2020, 152, 57–67.
- Sämfors, S.; Karlsson, K.; Sundberg, J.; Markstedt, K.; Gatenholm, P. Biofabrication of bacterial nanocellulose scaffolds with complex vascular structure. Biofabrication 2019, 11, 045010.
- Jabbari, F.; Babaeipour, V.; Bakhtiari, S. Bacterial cellulose-based composites for nerve tissue engineering. Int. J. Biol. Macromol. 2022, 217, 120–130.
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335.
- Fooladi, S.; Nematollahi, M.H.; Rabiee, N.; Iravani, S. Bacterial Cellulose-Based Materials: A Perspective on Cardiovascular Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2023, 9, 2949–2969.
- Hu, G.; Bao, L.; Li, G.; Chen, L.; Hong, F.F. Vascular cells responses to controlled surface structure and properties of bacterial nanocellulose artificial blood vessel after mercerization. Carbohydr. Polym. 2023, 306, 120572.
- Anton-Sales, I.; D’Antin, J.C.; Fernández-Engroba, J.; Charoenrook, V.; Laromaine, A.; Roig, A.; Michael, R. Bacterial nanocellulose as a corneal bandage material: A comparison with amniotic membrane. Biomater. Sci. 2020, 8, 2921–2930.
- Han, Y.; Li, C.; Cai, Q.; Bao, X.; Tang, L.; Ao, H.; Liu, J.; Jin, M.; Zhou, Y.; Wan, Y.; et al. Studies on Bacterial Cellulose/Poly (Vinyl Alcohol) Hydrogel Composites as Tissue-Engineered Corneal Stroma. Biomed. Mater. 2020, 15, 035022.
- Yang, J.; Du, M.; Wang, L.; Li, S.; Wang, G.; Yang, X.; Zhang, L.; Fang, Y.; Zheng, W.; Yang, G.; et al. Bacterial cellulose as a supersoft neural interfacing substrate. ACS Appl. Mater. Interfaces 2018, 10, 33049–33059.
- Hou, Y.; Wang, X.; Yang, J.; Zhu, R.; Zhang, Z.; Li, Y. Development and biocompatibility evaluation of biodegradable bacterial cellulose as a novel peripheral nerve scaffold. J. Biomed. Mater. Res. Part A 2018, 106, 1288–1298.
- Dydak, K.; Junka, A.; Szymczyk, P.; Chodaczek, G.; Toporkiewicz, M.; Fijałkowski, K.; Dudek, B.; Bartoszewicz, M. Development and biological evaluation of Ti6Al7Nb scaffold implants coated with gentamycin-saturated bacterial cellulose biomaterial. PLoS ONE 2018, 13, e0205205.
- Zuliani, C.C.; Damas, I.I.; Andrade, K.C.; Westin, C.B.; Moraes, A.M.; Coimbra, I.B. Chondrogenesis of human amniotic fluid stem cells in Chitosan-Xanthan scaffold for cartilage tissue engineering. Sci. Rep. 2021, 11, 3063.
- Bueno, V.B.; Bentini, R.; Catalani, L.H.; Barbosa, L.R.S.; Petri, D.F.S. Synthesis and characterization of xanthan–hydroxyapatite nanocomposites for cellular uptake. Mater. Sci. Eng. C 2014, 37, 195–203.
- Barbosa, R.M.; da Rocha, D.N.; Bombaldi de Souza, R.F.; Santos, J.L.; Ferreira, J.R.M.; Moraes, Â.M. Cell-Friendly Chitosan-Xanthan Gum Membranes Incorporating Hydroxyapatite Designed for Periodontal Tissue Regeneration. Pharmaceutics 2023, 15, 705.
- Souza, A.P.C.; Neves, J.G.; Navarro da Rocha, D.; Lopes, C.C.; Moraes, Â.M.; Correr-Sobrinho, L.; Correr, A.B. Chitosan/Xanthan membrane containing hydroxyapatite/Graphene oxide nanocomposite for guided bone regeneration. J. Mech. Behav. Biomed. Mater. 2022, 136, 105464.
- Souza, A.P.; Neves, J.G.; Navarro da Rocha, D.; Lopes, C.C.; Moraes, Â.M.; Correr-Sobrinho, L.; Correr, A.B. Chitosan/Xanthan/Hydroxyapatite-graphene oxide porous scaffold associated with mesenchymal stem cells for dentin-pulp complex regeneration. J. Biomater. Appl. 2023, 37, 1605–1616.
- Singh, A.; Muduli, C.; Senanayak, S.P.; Goswami, L. Graphite nanopowder incorporated xanthan gum scaffold for effective bone tissue regeneration purposes with improved biomineralization. Int. J. Biol. Macromol. 2023, 234, 123724.
- Piola, B.; Sabbatini, M.; Gino, S.; Invernizzi, M.; Renò, F. 3D Bioprinting of Gelatin–Xanthan Gum Composite Hydrogels for Growth of Human Skin Cells. Int. J. Mol. Sci. 2022, 23, 539.
- Decarli, M.C.; Seijas-Gamardo, A.; Morgan, F.L.C.; Wieringa, P.; Baker, M.B.; Silva, J.V.L.; Moraes, Â.M.; Moroni, L.; Mota, C. Bioprinting of Stem Cell Spheroids Followed by Post-Printing Chondrogenic Differentiation for Cartilage Tissue Engineering. Adv. Healthcare Mater. 2023, 12, 2203021.
- Revin, V.V.; Liyaskina, E.V.; Parchaykina, M.V.; Kuzmenko, T.P.; Urgaeva, I.V.; Revin, V.D.; Ullah, M.W. Bacterial Cellulose-Based Polymer Nanocomposites: A Review. Polymers 2022, 14, 4670.
- Salama, A.; Abouzeid, R.; Leong, W.S.; Jeevanandam, J.; Samyn, P.; Dufresne, A.; Bechelany, M.; Barhoum, A. Nanocellulose-Based Materials for Water Treatment: Adsorption, Photocatalytic Degradation, Disinfection, Antifouling, and Nanofiltration. Nanomaterials 2021, 11, 3008.
- Shi, R.-J.; Wang, T.; Lang, J.-Q.; Zhou, N.; Ma, M.-G. Multifunctional Cellulose and Cellulose-Based (Nano) Composite Adsorbents. Front. Bioeng. Biotechnol. 2022, 10, 891034.
- Shoukat, A.; Wahid, F.; Khan, T.; Siddique, M.; Nasreen, S.; Yang, G.; Ullah, M.W.; Khan, R. Titanium oxide-bacterial cellulose bioadsorbent for the removal of lead ions from aqueous solution. Int. J. Biol. Macromol. 2019, 129, 965–971.
- Hosseini, H.; Mousavi, S.M. Bacterial cellulose/polyaniline nanocomposite aerogels as novel bioadsorbents for removal of hexavalent chromium: Experimental and simulation study. J. Clean. Prod. 2021, 278, 123817.
- Mensah, A.; Lv, P.; Narh, C.; Huang, J.; Wang, D.; Wei, Q. Sequestration of Pb (II) Ions from Aqueous Systems with Novel Green Bacterial Cellulose Graphene Oxide Composite. Materials 2019, 12, 218.
- Revin, V.V.; Dolganov, A.V.; Liyaskina, E.V.; Nazarova, N.B.; Balandina, A.V.; Devyataeva, A.A.; Revin, V.D. Characterizing Bacterial Cellulose Produced by Komagataeibacter sucrofermentans H-110 on Molasses Medium and Obtaining a Biocomposite Based on It for the Adsorption of Fluoride. Polymers 2021, 13, 1422.
- Varsha, M.; Senthil Kumar, P.; Senthil Rathi, B. A Review on Recent Trends in the Removal of Emerging Contaminants from Aquatic Environment Using Low-Cost Adsorbents. Chemosphere 2022, 287, 132270.
- Wang, Y.; Yadav, S.; Heinlein, T.; Konjik, V.; Breitzke, H.; Buntkowsky, G.; Schneider, J.J.; Zhang, K. Ultra-light nanocomposite aerogels of bacterial cellulose and reduced graphene oxide for specific absorption and separation of organic liquids. RSC Adv. 2014, 4, 21553–21558.
- Zhu, H.-Y.; Fu, Y.-Q.; Jiang, R.; Jiang, J.-H.; Xiao, L.; Zeng, G.-M.; Zhao, G.-M.; Wang, Y. Adsorption Removal of congo Red onto Magnetic cellulose/Fe3O4/activated Carbon Composite: Equilibrium, Kinetic and Thermodynamic Studies. Chem. Eng. J. 2011, 173, 494–502.
- Galdino, C.J.S.; Maia, A.D.; Meira, H.M.; Souza, T.C.; Amorim, J.D.; Almeida, F.C.; Costa, A.F.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process. Biochem. 2020, 91, 288–296.
- Paulauskiene, T.; Uebe, J.; Ziogas, M. Cellulose aerogel composites as oil sorbents and their regeneration. PeerJ 2021, 9, e11795.
- Ieamviteevanich, P.; Daneshvar, E.; Eshaq, G.; Puro, L.; Mongkolthanaruk, W.; Pinitsoontorn, S.; Bhatnagar, A. Synthesis and Characterization of a Magnetic Carbon Nanofiber Derived from Bacterial Cellulose for the Removal of Diclofenac fromWater. ACS Omega 2022, 7, 7572–7584.
- Parizadeh, P.; Moeinpour, F.; Mohseni-Shahri, F.S. Anthocyanin-induced color changes in bacterial cellulose nanofibers for the accurate and selective detection of Cu(II) in water samples. Chemosphere 2023, 326, 138459.
- Sorze, A.; Valentini, F.; Dorigato, A.; Pegoretti, A. Development of a Xanthan Gum Based Superabsorbent and Water Retaining Composites for Agricultural and Forestry Applications. Molecules 2023, 28, 1952.
- Guimarães, D.T.; de Oliveira Barros, M.; de Araújo E Silva, R.; Silva, S.M.F.; de Almeida, J.S.; de Freitas Rosa, M.; Gonçalves, L.R.B.; Brígida, A.I.S. Superabsorbent bacterial cellulose film produced from industrial residue of cashew apple juice processing. Int. J. Biol. Macromol. 2023, 242, 124405.
- Sudirgo, M.M.; Surya, R.A.; Kristianto, H.; Prasetyo, S.; Sugih, A.K. Application of xanthan gum as coagulant-aid for decolorization of synthetic Congo red wastewater. Heliyon 2023, 9, e15011.
- Gbadamosi, A.; Patil, S.; Kamal, M.S.; Adewunmi, A.A.; Yusuff, A.S.; Agi, A.; Oseh, J. Application of Polymers for Chemical Enhanced Oil Recovery: A Review. Polymers 2022, 14, 1433.
- Gomes, N.O.; Carrilho, E.; Machado, S.A.S.; Sgobbi, L.F. Bacterial cellulose-based electrochemical sensing platform: A smart material for miniaturized biosensors. Electrochim. Acta 2020, 349, 136341.
- Yu, H.; Tian, Y.; Dirican, M.; Fang, D.; Yan, C.; Xie, J.; Jia, D.; Liu, Y.; Li, C.; Cui, M.; et al. Flexible, transparent and tough silver nanowire/nanocellulose electrodes for flexible touch screen panels. Carbohydr. Polym. 2021, 273, 118539.
- Kamel, S.; Khattab, T.A. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors 2020, 10, 67.
- Kotsiri, Z.; Vidic, J.; Vantarakis, A. Applications of Biosensors for Bacteria and Virus Detection in Food and Water–A Systematic Review. J. Environ. Sci. 2022, 111, 367–379.
- Torres, F.G.; Troncoso, O.P.; Gonzales, K.N.; Sari, R.M.; Gea, S. Bacterial cellulose based biosensors. Med. Devices Sens. 2020, 3, e10102.
- Faraco, T.A.; Fontes, M.L.; Paschoalin, R.T.; Claro, A.M.; Gonçalves, I.S.; Cavicchioli, M.; Farias, R.L.; Cremona, M.; Ribeiro, S.J.L.; Barud, H.D.S.; et al. Review of Bacterial Nanocellulose as Suitable Substrate for Conformable and Flexible Organic Light-Emitting Diodes. Polymers 2023, 15, 479.
- de Assis, S.C.; Morgado, D.L.; Scheidt, D.T.; de Souza, S.S.; Cavallari, M.R.; Ando Junior, O.H.; Carrilho, E. Review of Bacterial Nanocellulose-Based Electrochemical Biosensors: Functionalization, Challenges, and Future Perspectives. Biosensors 2023, 13, 142.
- Prilepskii, A.; Nikolaev, V.; Klaving, A. Conductive bacterial cellulose: From drug delivery to flexible electronics. Carbohydr. Polym. 2023, 313, 120850.
- Pan, X.; Li, J.; Ma, N.; Ma, X.; Gao, M. Bacterial cellulose hydrogel for sensors. Chem. Eng. J. 2023, 461, 142062.
- Zhou, Y.; Zhang, L.; Lin, X.; Lu, J.; Huang, Z.; Sun, P.; Zhang, Y.; Xu, X.; Li, Q.; Liu, H. Dual-network polyvinyl alcohol/polyacrylamide/xanthan gum ionic conductive hydrogels for flexible electronic devices. Int. J. Biol. Macromol. 2023, 233, 123573.
- Wu, Y.; Ren, Y.; Liang, Y.; Li, Y. Semi-IPN ionogel based on poly (ionic liquids)/xanthan gum for highly sensitive pressure sensor. Int. J. Biol. Macromol. 2022, 223, 327–334.
- Tomić, N.Z.; Ghodhbane, M.; Matouk, Z.; AlShehhi, N.; Busà, C. Enhancement of Self-Healing Efficacy of Conductive Nanocomposite Hydrogels by Polysaccharide Modifiers. Polymers 2023, 15, 516.
- Liu, Z.; Zhang, M.; Bhandari, B. Effect of gums on the rheological, microstructural and extrusion printing characteristics of mashed potatoes. Int. J. Biol. Macromol. 2018, 117, 1179–1187.
- Azam, R.S.M.; Zhang, M.; Bhandari, B.; Yang, C. Effect of different gums on features of 3D Printed Object Based on Vitamin-D enriched orange concentrate. Food Biophys. 2018, 13, 250–262.
- McCarthy, R.R.; Ullah, M.W.; Booth, P.; Pei, E.; Yang, G. The use of bacterial polysaccharides in bioprinting. Biotechnol. Adv. 2019, 37, 107448.
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D printing with cellulose materials. Cellulose 2018, 25, 4275–4301.
- Li, J.; Moeinzadeh, S.; Kim, C.; Pan, C.C.; Weale, G.; Kim, S.; Abrams, G.; James, A.W.; Choo, H.; Chan, C.; et al. Development and systematic characterization of GelMA/alginate/PEGDMA/xanthan gum hydrogel bioink system for extrusion bioprinting. Biomaterials 2023, 293, 121969.
- Li, J.; Shelby, T.; Alizadeh, H.V.; Shelby, H.; Yang, Y.P. Development and characterization of an automated active mixing platform for hydrogel bioink preparation. Int. J. Bioprint. 2023, 9, 705.
- Hassan, M.; Dave, K.; Chandrawati, R.; Dehghani, F.; Gomes, V.G. 3D printing of biopolymer nanocomposites for tissue engineering: Nanomaterials, processing and structure-function relation. Eur. Polym. J. 2019, 121, 109340.
- Cakmak, A.M.; Unal, S.; Sahin, A.; Oktar, F.N.; Sengor, M.; Ekren, N.; Gunduz, O.; Kalaskar, D.M. 3D printed polycaprolactone/gelatin/bacterial cellulose/hydroxyapatite composite scaffold for bone tissue engineering. Polymers 2020, 12, 1962.
- Aki, D.; Ulag, S.; Unal, S.; Sengor, M.; Ekren, N.; Lin, C.-C.; Yilmazer, H.; Ustundag, C.B.; Kalaskar, D.M.; Gunduz, O. 3D printing of PVA/hexagonal boron nitride/bacterial cellulose composite scaffolds for bone tissue engineering. Mater. Des. 2020, 196, 109094.
