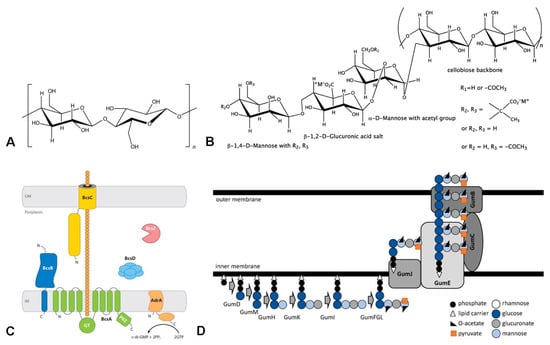
Xanthan is a heteropolysaccharide containing a cellulose-like backbone of β-1,4-linked glucose units substituted alternately with a trisaccharide side chain composed of two mannose units separated by a glucuronic acid. The internal mannose is mostly O-acetylated, and the terminal mannose can be substituted by a pyruvic acid residue (
Figure 1B). Due to the presenting glucuronic and pyruvic acid in the side chain, xanthan represents a highly charged polysaccharide with a very rigid polymer backbone. The content of pyruvate in xanthan ranges from 2.5–4.4%; this suggests that not every residue of the terminal D-mannopyranose in the side chain carries a pyruvate ketal group. Wu M. et al. obtained a genetically engineered
X. campestris strain CGMCC 15155, which produces high-viscosity xanthan with a pyruvate content of 8.69% [
59]. The low content of the pyruvyl group decreases the viscosity, while the high pyruvyl content contributes to the gel viscosity [
60]. The ratio of acetate in the xanthan molecule can also vary depending on the polymer sample. Higher acetyl content decreases the gelling capacity of xanthan gum in an aqueous solution [
60]. In addition, the hydrogen of the acetyl, pyruvic, and carboxyl groups in the D-glucuronic acid residue can be replaced by any cation. Thus, the pyranose sugar blocks in xanthan are not always structurally identical to each other, and the present acetic and pyruvic acids form an anionic polysaccharide. Xanthan has a high molecular mass of about 2 × 10
6 to 2 × 10
7 Da, which is influenced both by bacterium strains and fermentation conditions [
48]. The conformation of a polysaccharide macromolecule changes differently depending on pH, temperature, ionic strength, fermentation duration, medium composition, production method, etc. [
61]. Xanthan, in contrast to BC, is a water-soluble bacterial EPS and has high solubility in both cold and hot water. Xanthan molecules in aqueous solutions are prone to self-association, and a gel forms with an increase in the ionic strength of the solution or the concentration of the polysaccharide. It is a three-dimensional network formed from double helixes of xanthan linked by intermolecular hydrogen bonds [
62]. Xanthan has a pseudoplastic nature; that is, the viscosity inversely changes with the shear rate of a xanthan solution. Xanthan molecular structure and conformational state are closely associated with its rheology, stability, and function [
63,
64]. These properties enable it to be used as a thickening, dispersant, emulsifier, and viscous aqueous solution at low concentrations (0.05–1%). Xanthan has a high tolerance to deviations in the pH range of 2–12 and a high resistance to temperature changes. These properties confer industrial relevance and can explain the wide commercial EPS acceptance [
65]. The commercial demand is a key point stimulating studies to increase xanthan production on an industrial scale by sustainable processes to exploit the microorganism potential [
66,
67].
2. Applications of BC and Xanthan
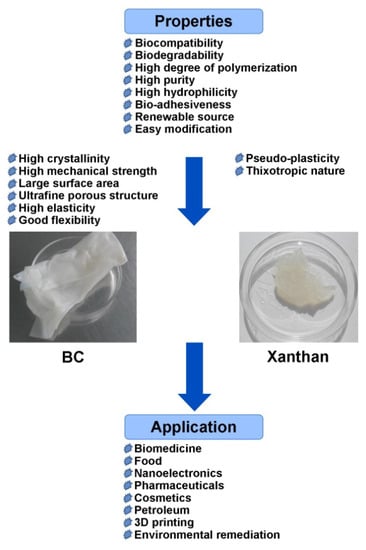
Despite a number of differences in their structure and properties, xanthan and BC have found wide application in similar fields of medicine, technology, and industry (
Figure 2). Their promotion is due to their unique beneficial properties such as biocompatibility, biodegradability, non-toxicity, a high degree of polymerization, water retention, and the ability for gelation. Thus, they can be commercially applied in food, pharmaceutical, cosmetic, chemical, textile, oil, and gas industries as thickeners, emulsifiers and suspension stabilizers, flocculants, and additives to improve the quality of different products. The biocompatibility and functional characteristics of BC and xanthan are key factors promoting their application in biomedicine, e.g., tissue engineering, wound dressing, and drug delivery systems. Recently, many reviews describing the use of BC [
7,
37,
52,
53,
54,
55,
56,
57,
236,
237,
238,
239,
240,
241,
242] and xanthan [
48,
63,
89,
243,
244,
245] in various fields have been published.
Figure 2. Properties and application of BC and xanthan.
Xanthan, which was discovered in the 1950s, belongs to one of the earliest marketed bacterial exopolysaccharides certified for food use in the USA [
246]. This polymer is environmentally friendly and non-toxic and therefore is used in the food industry as a thickener, stabilizer, and suspending agent in many foods and in the structure of biodegradable food packaging [
48,
79,
243,
244,
245,
246]. BC is also used as an additional thickener, a suspending or stabilizing agent in foods, or directly in food as an ingredient in fiber-enriched low-calorie and low-cholesterol diets, as well as the material for food packaging [
247,
248,
249,
250,
251,
252]. In 1992, the Food and Drug Administration (FDA) approved BC to be safe, and in 2019, the species
K. sucrofermentans was included in the list of Qualified Presumption of Safety (QPS) recommended biological agents and intentionally added to food [
249].
BC and xanthan are promising materials for biomedical applications since they are biocompatible polymers and not cytotoxic. BC-based materials for medicine have been produced for a relatively long time. Back in the early 1980s, the American pharmaceutical company Johnson & Johnson proposed using BC films to treat superficial wounds. Recently, several commercial medical BC-based materials have been obtained: Biofill
® (Curitiba, Brazil) and Bioprocess
® (Curitiba, Brazil) for burns and ulcer therapy, Gengiflex
® (Curitiba, Brazil) to treat periodontal diseases, Dermafill
® (Londrina, Brazil) for effective wound and ulcer healing, Membracel
® (Curitiba, Brazil) to treat venous leg ulcers and lacerations, xCell
® (New York, NY, USA) for chronic wounds therapy, EpiProtect
® (Royal Wootton Bassett, UK) for burn wounds, and Nanoskin
® incorporated with silver ions (São Carlos, Brazil) [
236,
253]. BC and xanthan have great potential to be used in medicine as a biomaterial for wound dressing [
254,
255,
256,
257,
258,
259,
260,
261,
262,
263,
264,
265,
266], drug delivery systems [
267,
268,
269,
270,
271,
272,
273,
274,
275,
276,
277,
278,
279,
280,
281,
282,
283,
284,
285,
286,
287,
288,
289], and tissue engineering [
290,
291,
292,
293,
294,
295,
296,
297,
298,
299,
300,
301,
302,
303,
304,
305,
306,
307,
308,
309,
310,
311,
312,
313,
314,
315,
316,
317,
318,
319,
320,
321,
322,
323,
324,
325,
326,
327,
328]. Recently, many reviews on BC-based materials for biomedical applications have been reported. This year, reviews by Qian et al. (2023) and Jadczak et al. (2023) summarized the state-of-the-art application of functional BC-based materials in biomedical fields [
52,
241]. Also, the recent review by Tang et al. (2022) discussed some of the biomedical applications that use BC, including wound healing, drug delivery, tissue engineering, and tumor cell and cancer therapy [
242]. The publications on using xanthan to obtain medical materials have appeared relatively recently. So, in the last two decades, researchers have taken an interest in its future use in drug delivery, tissue engineering, as well as biocomposites with regenerative and antibacterial properties [
261,
262,
263,
264,
265,
266,
274,
275,
276,
277,
278,
284,
285,
286,
287,
288,
289,
321,
322,
323,
324,
325,
326,
327,
328].
Wounds need an appropriate wound dressing to help prevent bacterial infection and accelerate wound closure. Wound dressing materials fabricated using biocompatible polymers have become quite relevant in medical applications [
267]. BC is a biopolymer that is commonly used for wound dressings due to its high biocompatibility, good flexibility, strong water-holding capacity, vapor permeability, elasticity, and non-toxicity [
254,
255]. Recently, a review by Horue et al. (2023) provided information on BC-based materials as dressings for wound healing [
53]. The authors reported the main characteristics of different BC structures such as films, membranes, fibers, etc., as well as recent advances in BC-based composites. Furthermore, the review by de Amorim et al. (2022) offers a summary of advances in the use of BC in composites and polymeric blends for drug delivery systems and wound healing [
258]. The review by Meng et al. (2023) introduces recent advances in BC-based antibacterial composites for the treatment of wound infection, including classification and preparation methods of composites, the mechanism of wound treatment, and commercial applications [
259]. Pasaribu et al. (2023) developed bioactive BC-based wound dressings for burns by impregnating collagen via an in situ method followed by immersing chitosan via an ex situ method into BC fibers [
260]. In vivo tests indicated that BC/collagen/chitosan wound dressing supported the wound healing process for second degree burns. Tang et al. (2022) developed hydrogel wound dressings using xanthan gum and polyacrylamide [
261]. With the combination of the polyacrylamide network and the xanthan network, the composite hydrogels showed high tensile strength, stretchability, excellent water uptake efficiency, outstanding biocompatibility, universal adhesion, and self-healing ability [
261]. Singh et al. (2022) developed polyvinyl alcohol copolymerized with xanthan gum/hypromellose/sodium carboxymethyl cellulose dermal dressings functionalized with biogenic nanostructured materials for antibacterial and wound healing applications [
262]. Recently, Gutierrez-Reyes et al. (2023) investigated novel hydrogels of semi-interpenetrating polymeric networks based on collagen and xanthan gum for wound healing applications [
263]. The increment of xanthan in the hydrogel (up to 20 wt.%) allows for improvement in the storage module, resistance to thermal degradation, and the slowing of the rate of hydrolytic and proteolytic degradation, allowing the encapsulation and controlled release of molecules such as ketorolac and methylene blue. Recently, Unalan et al. (2023) developed three-dimensional (3D)-printed sodium alginate–xanthan gum hydrogels containing phytotherapeutic agents with antioxidant and antibacterial activity as multifunctional wound dressings [
264]. Liang et al. (2023) prepared 3D-printed antibacterial hydrogels with benzyl isothiocyanate using xanthan gum, locust bean gum, konjac glucomannan, and carrageenan for burn wound healing [
265]. Alves et al. produced a thermo-reversible hydrogel composed of xanthan–konjac glucomannan (
Figure 5B) [
266]. The authors demonstrated the potential of composite hydrogels to improve the wound healing process by promoting fibroblast migration, adhesion, and proliferation [
266].
Drug delivery systems are used for the targeted delivery and/or controlled release of therapeutic drugs and have the advantage of reducing side effects, improving therapeutic effects, and possibly reducing drug doses [
267,
268]. Recently, EPSs have been considered as the ideal candidates for drug delivery systems due to their good biocompatibility, low immunogenicity, biodegradability, renewable sourcing, and easy modification [
269]. In recent years, interesting reviews have been published characterizing the EPS-based materials used in drug delivery systems [
269,
270,
271,
272]. For example, the review by Qiu et al. (2022) introduced a variety of polysaccharide-based nanocarriers such as nanoparticles, nanoliposomes, nanomicelles, nanoemulsions, and nanohydrogels for diabetes treatment [
270]. The review by Huo et al. (2022) summarized the latest research work on nanocellulose-based materials used in drug delivery [
271]. The review by Lunardi et al. (2021) provides a comprehensive overview of the procedures for modifying and functionalizing nanocellulose to obtain carriers in drug delivery systems [
272]. Chung et al. produced BC loaded with antibodies for optimizing checkpoint-blocking antibody delivery (
Figure 5C) [
273]. Recently, the review by Jadav et al. (2023) provided a comprehensive summary of current advances in xanthan modification to be used as an excipient in pharmaceutical formulation development, highlighting xanthan applicability to deliver various therapeutic agents such as drugs, genetic materials, proteins, and peptides [
274]. The important characteristics of xanthan for drug delivery systems are high stability at a low pH, which helps protect a drug in gastric fluid from degradation, and the ability to control the drug release rate by changing the pH and ionic strength of the release medium. Different forms of xanthan, such as hydrogels, matrix tablets, films, microspheres, and mucoadhesive patches, are synthesized to deliver drugs in various diseases [
274]. The review by Jadav et al. provides information on xanthan-based systems for the delivery of anti-diabetic drugs, anti-spasmodic drugs, immunosuppressive drugs, and drugs to treat inflammation, rheumatoid arthritis, gout, skin diseases, central nervous system-related disorders, obesity, glaucoma, and pulmonary diseases [
274]. Xanthan-based materials are used to deliver antibacterial [
275,
276], antiviral [
277], and antifungal [
278] drugs. Moreover, BC has been used to deliver antibacterial and antiseptic agents [
279]. BC and xanthan have been shown to be promising biomaterials for cancer treatment [
280,
281,
282,
283,
284,
285,
286,
287,
288]. For example, Cacicedo et al. combined a BC hydrogel and nanostructured lipid carriers to use as an implant for the local drug delivery of doxorubicin in cancer therapy [
281]. Zhang et al. developed BC-based composites with Fe
3O
4 magnetic doxorubicin-coated nanoparticles for breast cancer therapy [
282]. Microspheres, hydrogels [
284,
285,
286], pH-responsive nanoparticles [
287], and nanogels [
288] of xanthan were prepared for the delivery of anticancer drugs used to treat different cancers, including colon cancer. Recently, Anghel et al. (2023) developed novel xanthan-based materials as a delivery carrier for heparin [
289].
Recently, the fabrication of xanthan and BC-based scaffolds, including composites and blends with nanomaterials, and other biocompatible polymers has received particular attention owing to their desirable properties for tissue engineering. BC has a huge potential in tissue engineering due to its favorable mechanical properties, biocompatibility, high hydrophilicity, crystallinity, purity, high degree of polymerization, and ultrafine porous fibrous collagen-like structure [
290,
291,
292]. In the past few decades, many papers have been published on the use of BC in tissue engineering. Recently, the review by Raut et al. (2023) presented the latest modified/functionalized BC-based composites and blends as advanced materials in tissue engineering and summarized the latest updates on the production strategies and characterization of BC and its composites and blends [
292]. BC-based composites have proven to be promising materials in cartilage [
293,
294,
295,
296,
297,
298,
299,
300], bone [
301,
302,
303,
304,
305], soft tissue engineering such as blood vessels, adipose tissue, nerves, the liver, and skin [
306,
307,
308,
309,
310,
311,
312,
313,
314,
315,
316,
317,
318,
319,
320]. The review by Jabbari et al. (2022) discussed the importance and essential role of BC-based biomaterials in neural tissue regeneration and the effects of electrical stimulation on cellular behaviors [
312]. The review by Chen et al. (2022) summarized the application prospects of cellulose and its derivative-based hydrogels in biomedical tissue engineering [
313]. A recent review by Fooladi et al. (2023) discussed the application of BC-based materials for cardiovascular tissue engineering [
314]. Dydak et al. developed BC-coated Titanium-Aluminium-Niobium bone scaffold implants with low cytotoxicity against osteoblast and fibroblast cell cultures (
Figure 5E) [
320]. Zuliani et al. demonstrated that it is possible to differentiate stem cells from human amniotic fluid into chondrocytes when seeded directly in an efficient and low-cost chitosan-xanthan scaffold (
Figure 5D) [
321]. Bueno et al. obtained xanthan–hydroxyapatite hydrogel nanocomposites by precipitating hydroxyapatite in a xanthan aqueous solution. Nanocomposite hydrogels presented a porous structure and proved to be suitable for osteoblast growth [
322]. Recently, Barbosa et al. (2023) produced chitosan–xanthan composite membranes, incorporating hydroxyapatite to be used in guided tissue and bone regeneration, in particular for periodontal tissue regeneration [
323]. Souza et al. (2022) developed a chitosan–xanthan membrane associated with hydroxyapatite and different concentrations of graphene oxide for guided bone regeneration [
324]. Furthermore, Souza et al. (2023) synthesized polymeric scaffolds of chitosan/xanthan/hydroxyapatite-graphene oxide nanocomposites associated with mesenchymal stem cells for regenerative dentistry applications [
325]. Recently, Singh et al. (2023) fabricated biomaterial composed of xanthan and diethylene glycol dimethacrylate with impregnation of graphite nanopowder filler in their matrices for effective bone tissue regeneration purposes with improved biomineralization [
326]. Piola et al. developed a crosslinked 3D-printable hydrogel based on biocompatible natural polymers, gelatin, and xanthan gum at different percentages to be used both as a scaffold for human keratinocyte and fibroblast cell growth and as a wound dressing (
Figure 5F) [
327]. In another study, Decarli et al. (2023) reported a reproducible bioprinting process followed by a successful post-bioprinting chondrogenic differentiation procedure using human mesenchymal stromal cell spheroids encapsulated in a xanthan gum–alginate hydrogel [
328]. These results demonstrated a promising procedure to obtain 3D models for cartilage research and ultimately an in vitro proof-of-concept of their potential use as stable chondral tissue implants.
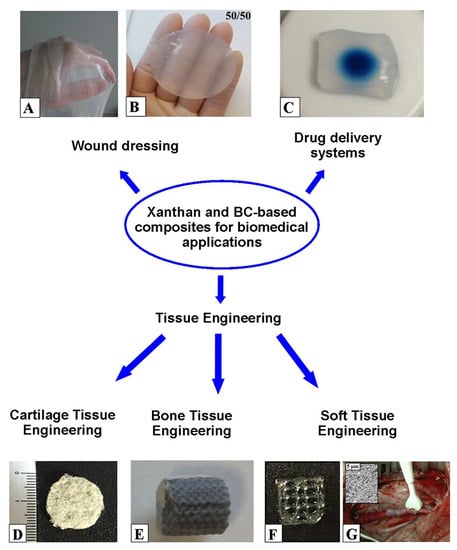
Figure 5 shows a schematic overview of biomedical applications of xanthan and BC-based composites.
Figure 5. Biomedical applications of xanthan and BC-based composites: BC gel film (
A); xanthan-konjac glucomannan composite hydrogel for wound healing (
B) (adapted from Ref. [
266] (open access)); BC loaded with IgG for optimizing checkpoint-blocking antibody delivery (
C) (adapted from Ref. [
273] (open access)); chitosan–xanthan scaffold for chondrocytes growth (
D) (adapted from Ref. [
321] (open access)); BC-coated Titanium-Aluminium-Niobium bone scaffold (
E) (adapted from Ref. [
320] (open access)); 3D-printed gelatin–xanthan composite hydrogel for growth of human skin cells (
F) (adapted from Ref. [
327] (open access)); BC graft implanted in the porcine carotid artery (
G) (adapted from Ref. [
307] (open access)).
BC has many advantages when used as an adsorbent, including a large surface area, high mechanical properties, biodegradability, and high reactivity due to the presence of hydroxyl groups on the surface, which enables its chemical modification to interact with various pollutants, depending on its nature [
46,
329]. A number of BC-based adsorbents have been obtained for removing hazardous metals [
330,
331,
332,
333], fluorine [
113], and organic pollutants, including dyes, pharmaceutical compounds, and petroleum products [
334,
335,
336,
337,
338,
339]. So, Salama et al. (2021) provided a comprehensive overview of the latest research results on nanocellulose-based materials for wastewater treatment, including adsorption, absorption, flocculation, photocatalytic decomposition, disinfection, etc., and discussed various approaches to their chemical modification [
329]. Parizadeh et al. (2023) developed an effective colorimetric sensor that detects copper (Cu(II)) ions in solutions and solid states using anthocyanin extract from black eggplant peels embedded in BC nanofibers [
340]. Xanthan functional groups are also able to bind heavy metals from aqueous solutions and effectively remove them. Xanthan can be used as a new green-based material to produce superabsorbents and remediate contaminated waters [
36,
243,
245]. A recent review by Balíkova et al. has emphasized the prospects for using xanthan as an environmentally friendly adsorbent for water disinfection [
36]. Sorze et al. (2023) have developed novel biodegradable hydrogel composites of xanthan and cellulose fibers that can be used both as soil conditioners and ground covers to stimulate plant growth and protect forests [
341]. Recently, Guimarães et al. (2023) received a superabsorbent BC film produced from industrial residues of cashew apple juice processing [
342]. Furthermore, bacterial EPSs have attracted interest for their applications, such as environmental bio-flocculants, because they are degradable and nontoxic. So, Sudirgo et al. (2023) showed xanthan to be a promising alternative as a coagulant aid for synthetic Congo red wastewater decolorization. The carboxylate group in xanthan could interact with polyaluminium chloride as the main coagulant, thus assisting the formation of larger flocs and resulting in improved coagulant performance [
343].
Xanthan is widely used in enhanced oil recovery technology because of its high viscosity, pseudoplastic behavior, salinity stability, temperature, and alkaline conditions [
344]. Furthermore, BC can be used for the microbial enhancement of oil recovery [
77]. Furthermore, BC and xanthan can be used in nanoelectronics (sensors, optoelectronic devices, flexible display screens, energy storage devices, and acoustic membranes) [
345,
346,
347,
348,
349,
350,
351,
352,
353,
354,
355,
356]. Ionic conductive hydrogels have received widespread attention as ideal candidates for flexible electronic devices. Conductive polymers such as polypyrrole, polythiophene, and polyaniline and nanomaterials such as carbon-based nanomaterials, metal nanoparticles, or nanowires are used in the synthesis of conductive hydrogels. The recent review by Prilepskii et al. (2023) presented some recent developments in electrically conductive BC-based composites for applications in numerous areas, including electrically conductive scaffolds for tissue regeneration, implantable and wearable biointerfaces, flexible batteries, sensors, and EMI shielding composites [
352]. The recent review by Pan et al. (2023) summarized the latest advances in BC hydrogel-based sensors, including strain, pH, electroactive, and thermal sensors [
353]. Recently, Zhou et al. (2023) developed dual-network polyvinyl alcohol/polyacrylamide/xanthan gum ionic conductive hydrogels for flexible electronic devices [
354]. Furthermore, Wu et al. (2022) prepared a novel ionogel with semi-interpenetrating poly (ionic liquids)/xanthan gum for highly sensitive pressure sensors [
355]. Currently, Tomić et al. (2023) are developing self-healing and self-adhesive conductive nanocomposite hydrogels by multiple and diverse coordination connections between various polysaccharide-based modifiers (xanthan, arabic gum, sodium carboxymethyl cellulose), the poly(vinyl alcohol) network, and different graphene-based fillers [
356]. Recently, xanthan and BC have received attention for their application in 3D printing technology [
327,
357,
358,
359,
360,
361,
362,
363,
364,
365]. Biopolymers as bioinks tend to be more profitable in terms of biocompatibility, nontoxicity, biodegradability, nonantigenicity, inertness, bio-adhesiveness, and adequate hemostasis compared to synthetic polymers. Xanthan has the required viscosity and shear thinning capacity, due to which it can function as a rheological modifier, thus improving 3D printing potential [
357,
358]. Recently, Li et al. (2023) developed a gelatin methacryloyl/alginate/polyethylene glycol dimethacrylate/xanthan gum hydrogel bioink system for extrusion bioprinting [
361]. Xanthan improved the viscosity of the hydrogel system and allowed easy extrusion at room temperature and demonstrated solubility in ionic solutions such as cell culture medium, which is essential for biocompatibility. They have also developed an automated active mixing platform which allows for the high-quality preparation of hydrogel bioinks [
362]. The use of BC and xanthan as bioinks for 3D printing has tremendous potential in tissue engineering and wound dressings [
264,
265,
328,
363,
364,
365]. Recently, Unalan et al. (2023) and Liang et al. (2023) prepared (3D)-printed hydrogels with xanthan for burn wound healing [
264,
265]. Cakmak et al. developed a 3D-printed BC/polycaprolactone/gelatin/hydroxyapatite composite scaffold for bone tissue engineering [
364]. Aki et al. also developed a 3D-printed PVA/hexagonal boron nitride/bacterial cellulose composite scaffold for bone tissue engineering [
365].