Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Male infertility is a condition that has always been less studied and known than female infertility. Male infertility is increasingly present and increasingly diagnosed. The worldwide denasality can only be slowed if awareness campaigns are implemented on all the diseases that can alter fertile potential, especially in young adolescents.
- pediatric
- infertility
- andrology
- preservation
1. Introduction
Infertility is defined as the inability to conceive after one year of regular unprotected intercourse. According to the World Health Organization, 48 million couples globally suffer from infertility, but the numbers may be higher when taking into account low-income countries, where access to fertility services may not be guaranteed. In 50% of cases infertility is attributable to male causes. In 20% of cases it is the sole responsible factor, while in 30–40% it is a co-responsible factor. There is also a reported declining trend in sperm count globally: from 1973 to 2018 the decline was 51.6%. Although the causes of this decline are not yet fully understood, a significant correlation has been found with increasing obesity rates, the Western diet, and exposure to environmental toxins. Male infertility is, in addition, associated with several medical conditions—in particular the association between infertility and testicular cancer, cardiovascular disease, autoimmune diseases, and genetic disorders is well known. Male infertility is a condition that has always been less studied and known than female infertility; the problem of infertility often becomes apparent in adulthood, following tests performed in cases of failure to conceive naturally, when the only viable strategy is through medically-assisted procreation techniques [1][2][3][4][5][6].
The causes of male infertility can be divided into four categories:
-
Endocrine and systemic disorders with hypogonadotropic hypogonadism (5–15% of cases)
-
Primary testicular defects of spermatogenesis (70–80% of cases)
-
Defects in sperm transport (2–5% of cases)
-
Idiopathic male infertility (10–20% of cases)
Any disease that can be related to hypogonadotropic hypogonadism, with a deficiency of hypothalamic/pituitary function, can result in a defect in spermatogenesis, failing stimulation with GnRH on the pituitary or gonadotropins on the testis. The consequence of this is the absence of hormonal stimulation on the testis related to spermatogenesis [3][4][5][6][7].
2. Spermatogenesis and Andrological Diseases
The term spermatogenesis refers to a set of processes that occur in the seminiferous tubules and result in the production of mature male gametes (Figure 1). The processes are, in temporal order: proliferation of spermatogonia; differentiation of spermatogonia into spermatocytes; meiotic division of spermatocytes into spermatids; release of mature spermatozoa into the tubular lumen. The main cellular actors involved in spermatogenesis are peritubular myoid cells, Leydig cells, Sertoli cells, and germ cells.
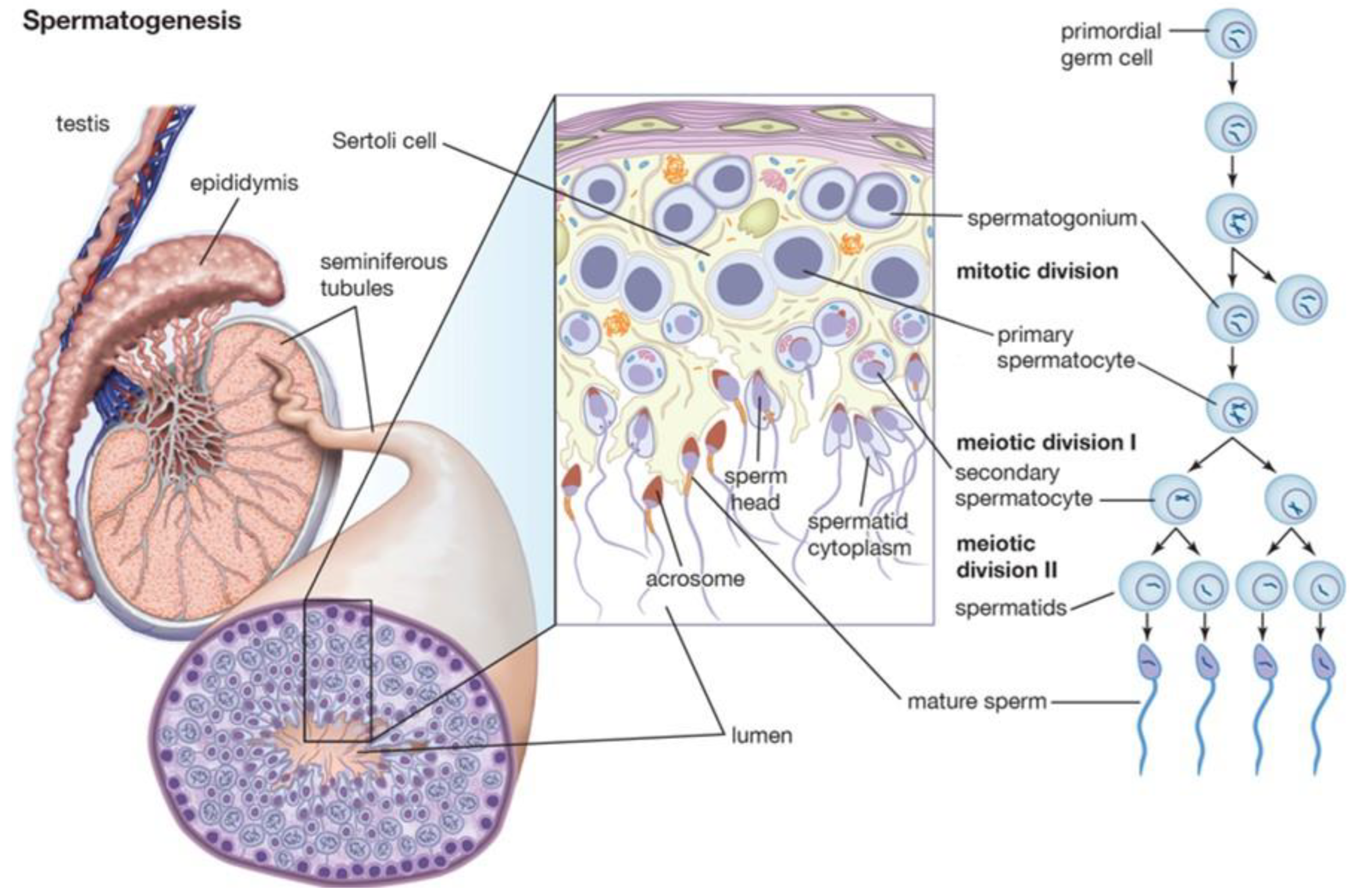
Figure 1. Spermatogenesis and hormonal control.
Peritubular myoid cells are cells of mesenchymal origin that provide support to the seminiferous tubules and, due to contractile properties, ensure sperm propulsion through the tubules. Another important function of these cells is the maintenance of the blood–testicular barrier, a structure responsible for separating the basal and adluminal compartments, ensuring immune tolerance in the confrontation of germ cells. Several factors control the activity of these cells, most notably androgen hormones.
Leydig cells are cells of mesenchymal origin, located between blood vessels and seminiferous tubules. Their main function is the production of testosterone, in amounts of about 3–10 mg/day in adults. Intratesticular testosterone levels are on the order of 100 times higher than in blood, and the maintenance of these concentrations is necessary for proper spermatogenesis. Leydig cells are, in addition, the main source of estradiol in humans. The maintenance of a constant ratio of testosterone to estrogen is involved in proper testicular function. The most important factor involved in Leydig cell function is luteinizing hormone (LH). This hormone induces steroidogenesis and inhibits cell apoptosis.
Sertoli cells are supportive cells that drive spermatogenesis. They occupy 20% of the epithelium of the seminiferous tubules and are distinguished from germ cells by their irregular shape. The cytoplasmic offshoots of Sertoli cells support germ cells at different maturational stages. A single Sertoli cell can support about 30–50 germ cells. One of the main products secreted by Sertoli cells is androgen-binding protein (ABP). This protein binds testosterone and dihydrotestosterone with high affinity, ensuring its high concentration in the extracellular space, which is critical for germ cell function. Sertoli cells also act as macrophages, clearing through phagocytosis the extracellular space of spermatid remnants or senescent germ cells. The regulation of Sertoli cell function occurs, primarily, by follicle-stimulating hormone (FSH), androgen hormones, and insulin.
FSH, produced by the pituitary upon stimulation of hypothalamic gonadotropin-releasing hormone (GnRH), activates several intracellular signal transduction pathways that regulate spermatogenesis. Androgen hormones play a role in meiosis and spermatid maturation. Insulin regulates the production of lactate, the main source of energy for germ cells. The latter finding could explain the defects in spermatogenesis that are evident in patients with type I diabetes.
Germ cells are the main actors in spermatogenesis. They are the only human cells that can carry out meiosis. They are distributed in an organized manner in the seminiferous tubules, with the most mature cells occupying the most superficial layers; germ cells are the main actors in spermatogenesis. They are the only human cells that can carry out meiosis. They are distributed in an organized manner in the seminiferous tubules, with the most mature cells occupying the most superficial layers. Primordial germ cells occupy the gonadal crest between 3 to 5 weeks of age, where they differentiate into gonocytes and arrest at the G0 stage of the cell cycle. Several factors, including glial-derived neurotrophic factor (GDNF), fibroblast growth factor (FGF), colony stimulating factor 1 (CSF1), retinoic acid, and DNA methylation, play a role in primordial germ cell differentiation. Between birth and 6 months of age, gonocytes differentiate into spermatogonia. Spermatogonia remains quiescent until puberty, when differentiative processes into spermatozoa begin. Spermatogonia has the dual role of differentiation into spermatozoa by meiosis and self-renewal by mitosis. It is accepted that Sertoli cell-derived factors regulate the fate of spermatogonia toward differentiation, self-renewal, or apoptosis. Primary spermatocytes, produced by mitosis of spermatogonia, move to the adluminal compartment, where they proceed to maturation by DNA recombination. Failure of this stage may explain the cases of aneuploidia that are evidenced by genetic testing in infertile men. After meiosis 1, two secondary spermatocytes are formed, with haploid chromosomal equipment. During meiosis 2, two spermatids are formed from secondary spermatocytes. During spermiogenesis, a series of cytoplasmic and nuclear changes occur, through which spermatozoa are obtained. These changes include acrosome formation, DNA condensation, and tail formation. At the conclusion of the process, the spermatozoon is released into the tubular lumen, at the stage known as spermiation.
Sperm pathology refers to alterations in quality or quantity. Diagnosis is mainly based on seminal fluid analysis. Quantitative alterations affecting male fertility are oligozoospermia (few sperm produced) and azoospermia (no sperm produced). Qualitative alterations potentially affect every portion of the cell, with the most significant from the point of view of fertility being alterations in the tail (impair motility) and head (impair fertilizing capacity).
Identifying normal values for spermiogram parameters is not easy, precisely because individual patients’ values tend to vary widely over several days. In addition, several studies have shown how there are important geographic differences in test results and how even within the same population individual tests can have high variability. These changes are critical, especially for the study and management of many andrological diseases that may alter fertile potential. After understanding all the fundamental passages of spermatogenesis and its regulation, it is possible to understand how various alterations created by external pathologies can affect the correct mechanism. Hormonal alterations will be the cause of the failure of Sertoli and Leydig cells to function, and alterations in temperature and the production of free radicals directly generated by stress on the testis will be the cause of the failure of spermatozoa to mature, and genetic alterations will be associated with more or less complete alterations in molecular regulatory mechanisms [2][3][4][5][6][7].
This entry is adapted from the peer-reviewed paper 10.3390/life13091934
References
- Nassau, D.E.; Chu, K.Y.; Blachman-Braun, R.; Castellan, M.; Ramasamy, R. The pediatric patient and future fertility: Optimizing long-term male reproductive health outcomes. Fertil. Steril. 2020, 113, 489–499.
- Mulder, R.L.; Font-Gonzalez, A.; Green, D.M.; Loeffen, E.A.H.; Hudson, M.M.; Loonen, J.; Yu, R.; Ginsberg, J.P.; Mitchell, R.T.; Byrne, J.; et al. Fertility preservation for male patients with childhood, adolescent, and young adult cancer: Recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e57–e67.
- Gertosio, C.; Magistrali, M.; Musso, P.; Meazza, C.; Bozzola, M. Fertility Preservation in Pediatric Oncology Patients: New Perspectives. Adolesc. Young Adult Oncol. 2018, 7, 263–269.
- Delgouffe, E.; Braye, A.; Goossens, E. Testicular Tissue Banking for Fertility Preservation in Young Boys: Which Patients Should Be Included? Front. Endocrinol. 2022, 13, 854186.
- Brannigan, R.E.; Fantus, R.J.; Halpern, J.A. Fertility preservation in men: A contemporary overview and a look toward emerging technologies. Fertil. Steril. 2021, 115, 1126–1139.
- Goossens, E.; Jahnukainen, K.; Mitchell, R.T.; van Pelt, A.; Pennings, G.; Rives, N.; Poels, J.; Wyns, C.; Lane, S.; Rodriguez-Wallberg, K.A.; et al. Fertility preservation in boys: Recent developments and new insights. Hum. Reprod. Open 2020, 2020, hoaa016.
- Koo, J.; Grom-Mansencal, I.; Howell, J.C.; Rios, J.M.; Mehta, P.A.; Davies, S.M.; Myers, K.C. Gonadal function in pediatric Fanconi anemia patients treated with hematopoietic stem cell transplant. Haematologica 2023, 108, 2358–2368.
This entry is offline, you can click here to edit this entry!
