Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Marine soft corals are prolific sources of various natural products that have served as a wealthy reservoir of diverse chemical scaffolds with potential as new drug leads. The genus Litophyton contains almost 100 species but only a small proportion of them has been chemically investigated, which calls for more attentions from global researchers.
- soft coral
- Litophyton
- secondary metabolites
- terpenes
- bioactivities
- cytotoxicity
1. Introduction
More than two-thirds of the Earth’s surface is covered by oceans, which harbor a vast array of creatures, including plants, animals, and microbes. Since the ancient times, marine organisms have been used as sources of foods [1], cosmetic ingredients [2], and drugs [3], which are hotspots for global researchers nowadays [4]. Continuous studies focused on the secondary metabolites derived from marine environments, resulting in a rapid expansion of marine natural products [5]. These substances displayed a wide spectrum of potential pharmacological effects, including antivirus [6], anti-osteoclastogenesis [7], antimicrobial [8], and antitumor [9]. To date, almost 20 drugs from marine sources are in clinical use [10].
The marine soft coral genus Litophyton belongs to the family Nephtheidae, order Alcyonacea, subclass Octocorallia. It might be worth pointing out the taxonomic relationship between the genera Nephthea and Litophyton, both of which are in the same family Nephtheidae. In 2016, the genus Nephthea was synonymized with the genus Litophyton due to their identical characteristics in terms of mitochondrial DNA molecular information and morphology (including features such as bone needle, tentacle shape, polyp, and stem) [11][12]. Currently, the genus Litophyton consists of nearly 100 species, according to the Word Register of Marine Species (WoRMS) [13]. They are widely distributed throughout tropical and temperate waters, such as the South China Sea [14], Red Sea [11], as well as other waters of the Indo-Pacific Ocean [15][16][17].
The alcyonarian Litophyton viridis was observed to provide chemical protection for the fish Abudefduf leucogaster [18]. In addition to the ecological role, the extracts of several soft corals of the genus Litophyton have been biologically screened and showed a variety of potent bioactivities, such as antioxidant [19], genotoxic [20], cytotoxic [19][21][22], HIV-1 enzyme inhibitory [21], antibacterial [22], anti-inflammatory [23], antifungal [24], and wound healing [25] activities. Chemical investigations on Litophyton soft corals were carried out by researchers worldwide and revealed that soft corals of the genus Litophyton are prolific producers of bioactive secondary metabolites.
2. Classification of Secondary Metabolites from the Genus Litophyton
Since the early reports of novel cembrane diterpenes from the soft corals Nephthea sp. [26] and L. viridis [27] in the beginning of 1970s, many research groups around the world have carried out chemical investigation of the genus Litophyton, resulting in fruitful achievements. For instance, two uncommon bis-sesquiterpenes, dikelsoenyl ether and linardosinene H, were encountered during the research of two alcyonarians, Nephthea erecta [28] and Litophyton nigrum [29], respectively. Up to July 2023, a total of 175 secondary metabolites have been isolated and characterized in Litophyton corals during almost 50 years of research (Table S1). These chemical compounds can be structurally classified as sesquiterpenes, sesquiterpene dimers, diterpenes, norditerpenes, tetraterpenes, meroterpenes, steroids, ceramides, pyrimidines, peptides, prostaglandins, γ-lactones, fatty acids, glycerol ethers, and selenides. In the following subsections, these compounds were further grouped under different categories based on their structural features. Among them, the ceramides, pyrimidines, and peptides were placed under one category, ‘nitrogen-containing metabolites’. The pack of ‘lipids’ comprise prostaglandins, γ-lactones, fatty acids, and glycerol ethers. Other metabolites include selenides.
3. Sesquiterpenes
This was a large cluster of terpenes obtained from the genus Litophyton with an account of 38 compounds. These compounds possessed a variety of carbon frameworks, which could be further classified into 14 categories: bicyclogermacrane, sec-germacrane, guaiane, pseudoguaiane, himachalene, eudesmane, seco-eudesmane, tri-nor-eudesmane, eremophilane, nardosinane, nornardosinane, neolemnane, seconeolemnane, and kelsoane (Figure 1). This diversity of skeletons makes sesquiterpenes the most interesting type of natural products from this genus. The different sesquiterpenes were distributed in four species, Litophyton arboreum, L. nigrum, Litophyton setoensis, Nephthea erecta, and an unclearly identified Nephthea sp., which inhabited different marine environments including the Red Sea, South China Sea, and the waters around Indonesia and Taiwan.
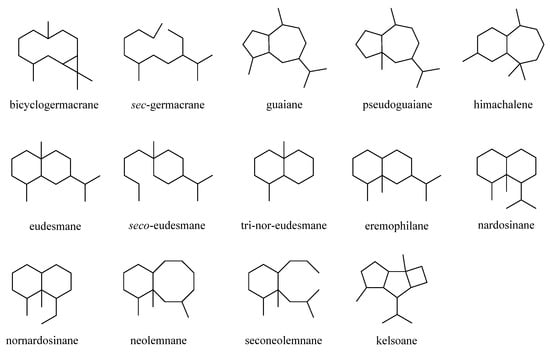
Figure 1. Carbon frameworks of the sesquiterpenes reported from soft corals of the genus Litophyton.
3.1. Bicyclogermacrane Sesquiterpenes
Chemical investigation of the soft coral L. arboreum, which was collected near Bali, Indonesia, yielded the sesquiterpene (−)-bicyclogermacrene (1) [30] (Figure 2). This compound exhibited low antiproliferative activities against the cell lines L-929 and K-562 with GI50 values of 186 and 200 μM, respectively, and low cytotoxic effect against the HeLa cell line with CC50 of 182 μM.
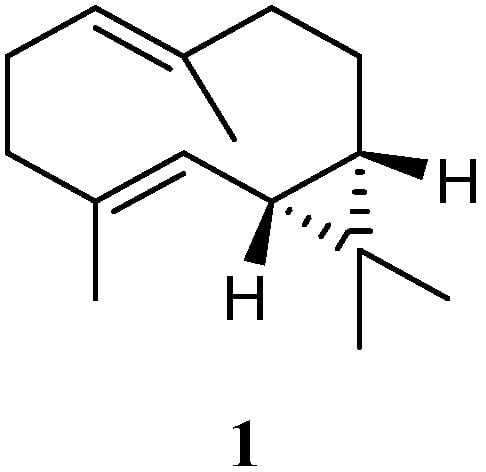
Figure 2. Chemical structure of the bicyclogermacrane sesquiterpene isolated from soft corals of the genus Litophyton.
3.2. Sec-Germacrane Sesquiterpenes
Very recently, Ahmed et al. [31] carried out chemical investigation of the Red Sea specimen L. arboreum, which was collected at Neweba, Egypt. The acyclic sesquiterpene (2E,6E)-3-isopropyl-6-methyl-10-oxoundeca-2,6-dienal (2) was found from this sample, which possessed a sec-germacrane nucleus (Figure 3). Anti-malarial bioassays disclosed the isolate 2 was active against chloroquine-sensitive (D6) and chloroquine-resistant (W2) strains of Plasmodium falciparum with IC50 values of 3.7 and 2.2 mg/mL, respectively. In addition, the metabolite 2 was non-toxic to the Vero cell line at the concentration of 4.76 mg/mL. These findings demonstrated that sesquiterpene 2 could be developed as an anti-malarial lead compound that is highly safe in the range of tested concentrations.
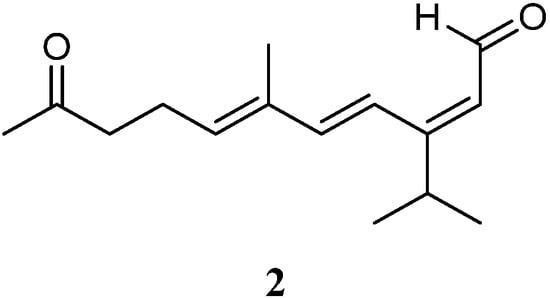
Figure 3. Chemical structure of the sec-germacrane sesquiterpene from soft corals of the genus Litophyton.
3.3. Guaiane Sesquiterpenes
Interestingly, the guaiane sesquiterpenes were frequently encountered in the Red Sea soft coral L. arboreum.
Bioassay-guided fractionation of the Red Sea alcyonarian L. arboreum by Ellithey et al., which was collected at Sharm El-Sheikh, Egypt, yielded three guaiane sesquiterpenes alismol (3), 10-O-methyl alismoxide (4), and alismoxide (5) [32] (Figure 4). Compound 3 showed potent inhibitory activity against HIV-1 protease receptor with IC50 of 7.2 µM, compared to the positive control, which had IC50 of 8.5 μM. A molecular docking study disclosed the hydrogen bond between 3 and the amino acid residue Asp 25 in the hydrophobic receptor pocket with a score of −11.14. Meanwhile, sesquiterpenes 3 and 4 showed moderate cytotoxic activities against the cell lines HeLa (IC50 30 and 38 μM, respectively) and Vero (IC50 49 and 49.8 μM, respectively). Moreover, 4 exhibited moderate cytotoxicity against the U937 cell line with IC50 of 50 µM. However, 5 was judged as inactive against the above-mentioned cell lines (all IC50 > 100 µM). In a further study, compounds 2 and 5 demonstrated cytostatic action in HeLa cells, revealing potential use in virostatic cocktails. In Ellithey’s continual study [33], alismol (3) showed promising cytotoxic effects against the cancer cell lines HepG2, MDA and A549 (IC50 4.52, 7.02, and 9.23 μg/mL, respectively).
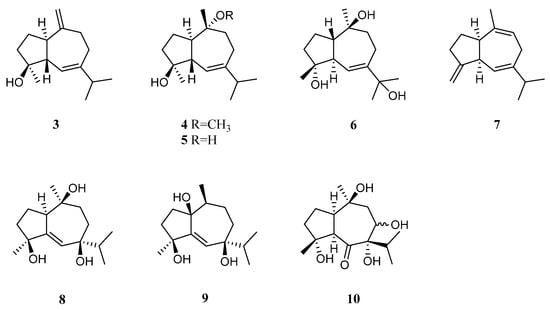
Figure 4. Chemical structures of the guaiane sesquiterpenes from soft corals of the genus Litophyton.
Hawas’s group reported the presence of alismol (3) in a Red Sea specimen of L. arboreum collected off the coast of Jeddah, Saudi Arabia, together with another guaiane sesquiterpene alismorientol B (6) [34] (Figure 4). These two secondary metabolites were subjected to antimicrobial and cytotoxic bioassays. As a result, metabolites 3 and 6 showed weak to strong antibacterial activities against Escherichia coli ATCC 10536, Pseudomonas aeruginosa NTCC 6750, Bacillus cereus ATCC 9634, Bacillus subtilis ATCC6633, and Staphylococcus aureus ATCC5141 with MIC values ranging from 10.4 to 1.3 μg/mL. Here, compound 6 had significant activity against B. cereus ATCC 9634 with MIC of 1.3 μg/mL. Compounds 3 and 6 exhibited weak to moderate antifungal activities against Candida albicans and Aspergillus niger with MIC values ranging from 10.1 to 6.0 μg/mL. Moreover, they displayed cytotoxic effects against the cell lines MCF-7, HCT-116, and HepG2, with IC50 ranging from 4.32 to 44.52 μM. Here, compound 6 showed the most potent cytotoxic effect against MCF-7 cells with IC50 of 4.32 μM. Additionally, Hawas’s group evaluated the methanolic extract of the above-mentioned soft coral for its in vivo genotoxicity and antigenotoxicity against the mutagenicity induced by the anticancer drug cyclophosphamide [20]. The extract was found to be safe and nongenotoxic at 100 mg/kg b. wt. Moreover, the mice group of cyclophosphamide pretreated with the extract (100 mg/kg b. wt.) showed significant reduction in the percentage of chromosomal aberrations induced in bone marrow and mouse spermatocytes.
The existence of alismoxide (5) was shown in the Egyptian Red Sea L. arboreum collection from Hurghada by Mahmoud et al. [35]. In the anticancer bioassays, sesquiterpene 5 displayed no cytotoxic activities against the cell lines A549, MCF-7, and HepG2 (all IC50 > 100 µmol/mL). The co-existence of alismol (3) and alismoxide (5) as well as an undescribed sesquiterpene, litoarbolide A (7), and three known analogues 4α,7β,10α-trihydroxyguai-5-ene (8), leptocladol B (9), and nephthetetraol (10) (Figure 4) in another Egyptian Red Sea L. arboreum specimen from Neweba, was revealed by Ahmed et al.’s work [31]. Viewing from the perspective of their structures, litoarbolide A (7) was supposed to be the biosynthetic precursor to other sesquiterpenes, which could be generated via further post-translational modifications. The anti-malarial properties of substances 7–10 were evaluated. However, only compounds 9 and 10 exhibited anti-malarial activities against chloroquine-resistant P. falciparum W2 with IC50 values of 4.3 and 3.2 mg/mL, respectively.
Guaiane sesquiterpenes 10-O-methyl alismoxide (4) and alismoxide (5) were also obtained from the octocoral Nephthea sp. by Hegazy et al., which was collected from the Egyptian Red Sea off the coast of Hurghada [36]. These two metabolites showed cytotoxicity against the cell line MCF-7 (IC50 85.5 and 151.9 μg/mL, respectively).
3.4. Pseudoguaiane Sesquiterpenes
A new pseudoguaiane-type sesquiterpene named litopharbol (11) (Figure 5) was isolated from the methanolic extract of the Saudi Arabian Red Sea soft coral L. arboreum by Hawas’s group [34]. Its structure was determined through the elucidation of NMR data. Compound 11 exhibited a wide spectrum of antibacterial activities against Gram-negative bacteria E. coli ATCC 10536 and P. aeruginosa NTCC 6750, as well as Gram-positive bacteria B. cereus ATCC 9634, B. subtilis ATCC6633, and S. aureus ATCC5141 with MIC values ranging from 1.8 to 9.6 μg/mL. Among these bacteria, 11 showed significant activity against B. cereus ATCC 9634 with an MIC of 1.8 μg/mL. In addition, this sesquiterpene exhibited weak antifungal activities against C. albicans and A. niger with MIC values of 12.5 and 12.9 μg/mL, respectively. Moreover, it displayed cytotoxic effects against cell lines MCF-7, HCT-116, and HepG2 with IC50 values of 9.42, 26.21, and 38.92 μM, respectively. In Hawas’s continual study, litopharbdiol (12) was identified, which shared the same carbon framework with 11 [20] (Figure 5). However, no bioassay for this compound was reported in the article.
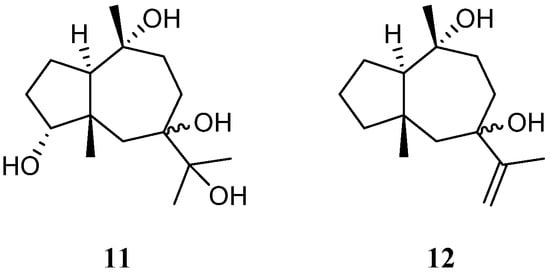
Figure 5. Chemical structures of the pseudoguaiane sesquiterpenes from soft corals of the genus Litophyton.
3.5. Himachalene Sesquiterpenes
Purification of the CH2Cl2/MeOH extract of Saudi Arabian Red Sea alcyonarian L. arboreum yielded a new himachalene-type sesquiterpene 3α,6α-epidioxyhimachal-1-ene (13) (Figure 6), which showed antiproliferative effects toward three different cancer cell lines MCF-7, HCT116, and HepG-2 [37]. (It might be worth pointing out that no specific data of the bioassay results were provided in this article).
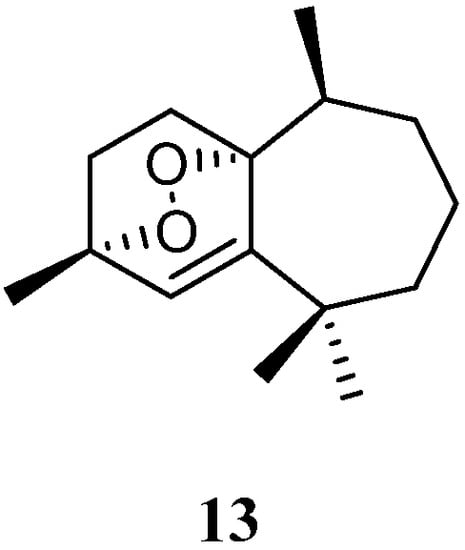
Figure 6. Chemical structure of the himachalene sesquiterpene from soft corals of the genus Litophyton.
3.6. Eudesmane Sesquiterpenes
The n-hexane-chloroform (1:1) fraction of the Egyptian Red Sea L. arboreum sample exhibited cytotoxicity towards the A549 cell line (IC50 22.6 mg/mL) [35]. The subsequent bioassay-guided isolation yielded a eudesmane sesquiterpene 5β,8β-epidioxy-11-hydroxy-6-eudesmene (14) (Figure 7). Compound 14 exerted noticeable activity against the A549 cell line (IC50 67.3 µmol/mL) compared to etoposide as standard cytotoxic agent (IC50 48.3 µmol/mL). However, this compound did not show cytotoxic effects against cell lines MCF-7 and HepG2 (both IC50 > 100 µmol/mL).
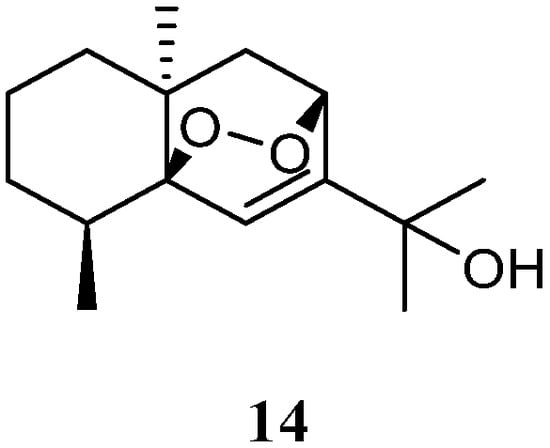
Figure 7. Chemical structure of the eudesmane sesquiterpene from soft corals of the genus Litophyton.
3.7. Seco-Eudesmane Sesquiterpenes
In the above-mentioned study [35], a seco-eudesmane sesquiterpene chabrolidione B (15) (Figure 8) was co-isolated. However, compound 15 was judged as inactive against the cell lines A549, MCF-7, and HepG2 (all IC50 > 100 µmol/mL).
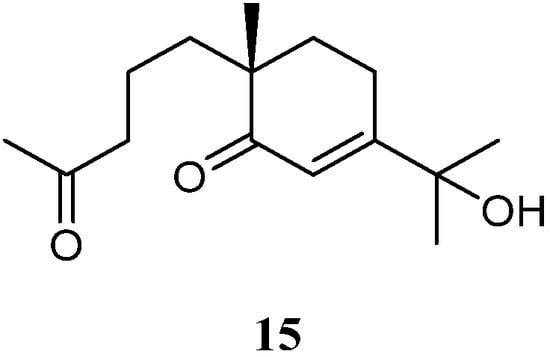
Figure 8. Chemical structure of the seco-eudesmane sesquiterpene from soft corals of the genus Litophyton.
3.8. Tri-Nor-Eudesmane Sesquiterpenes
The methanolic extract of the Saudi Arabia Red Sea L. arboreum collection harbored two tri-nor-eudesmane sesquiterpenes teuhetenone A (16) and calamusin I (17) [34] (Figure 9). Interestingly, these two nor-sesquiterpenes 16 and 17 displayed a wide spectrum of bioactivities. In the antibacterial bioassays, they showed moderate to strong activities against E. coli ATCC 10536, P. aeruginosa NTCC 6750, B. cereus ATCC 9634, B. subtilis ATCC6633, and S. aureus ATCC5141 with MIC values ranging from 10.9 to 1.2 μg/mL. Here, 16 exhibited the most potent activity against E. coli ATCC 10536 with an MIC of 1.9 μg/mL, and 17 displayed the most potent activity against P. aeruginosa NTCC 6750 with an MIC of 1.2 μg/mL. In the antifungal biotests, they exhibited weak to moderate activities against C. albicans and A. niger with MIC values ranging from 7.4 to 3.2 μg/mL. In the cytotoxic experiments, they displayed cytotoxic effects against cell lines MCF-7 and HepG2 with IC50 ranging from 6.43 to 39.23 μM. In addition, the methanolic extract of the Egyptian Red Sea L. arboreum sample yielded another tri-nor-eudesmane sesquiterpene 7-oxo-tri-nor-eudesm-5-en-4β-ol (18) [35] (Figure 9). However, this nor-sesquiterpene 18 did not show cytotoxic activities against the cell lines A549, MCF-7, and HepG2 (all IC50 > 100 µmol/mL).
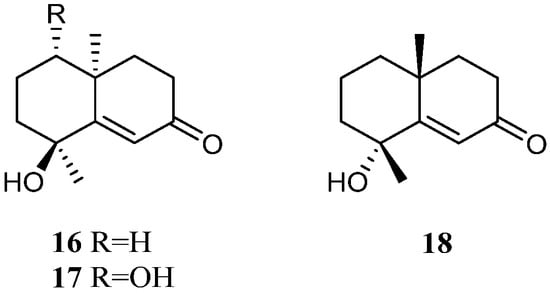
Figure 9. Chemical structures of the tri-nor-eudesmane sesquiterpenes from soft corals of the genus Litophyton.
3.9. Eremophilane Sesquiterpenes
11,12-Dihydroxy-6,10-eremophiladiene (19) (Figure 10) was obtained from the soft coral L. nigrum, using a structure-oriented HR-MS/MS approach [29]. This alcyonarian specimen was collected at Xisha Islands, Hainan, China. However, no bioassays were performed due to its scarcity.
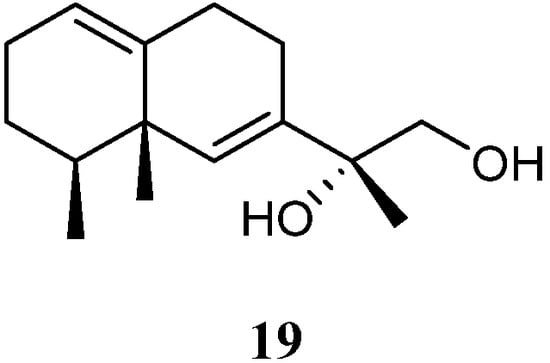
Figure 10. Chemical structure of the eremophilane sesquiterpene from soft corals of the genus Litophyton.
3.10. Nardosinane Sesquiterpenes
Interestingly, the South China Sea soft coral L. nigrum is a rich source of nardosinane sesquiterpenes.
The chemical investigation of the Xisha collection by Yang et al. afforded two new terpenes linardosinenes B (20) and C (21) [14] (Figure 11). These two compounds were evaluated for cytotoxities against different cell lines. Sesquiterpene 20 exhibited cytotoxic effect against the THP-1 cell line with IC50 of 59.49 μM, while compound 21 displayed cytotoxicities against the cell lines SNU-398 and HT-29 with IC50 of 24.3 and 44.7 μM, respectively. In their continual study on the Xisha sample, four additional new secondary metabolites linardosinenes D–G (22–25) (Figure 11) were obtained [38]. All metabolites exhibited weak inhibitory effect against bromodomain-containing protein 4 (BRD4), a promising therapeutic target in various human diseases, at a concentration of 10 μM with inhibitory rates ranging from 15.8% to 18.1%.
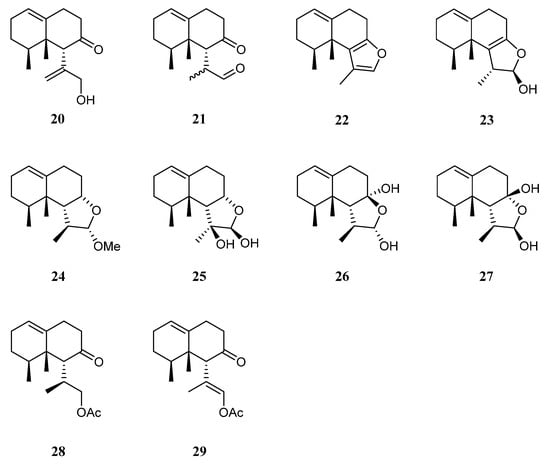
Figure 11. Chemical structures of the nardosinane sesquiterpenes from soft corals of the genus Litophyton.
Using a structure-oriented HR-MS/MS approach, an undescribed sesquiterpene linardosinene I (26), along with its known 7β,12α-epimer lemnal-l(l0)-ene-7β,12α-diol (27) (Figure 11) were isolated from Xisha alcyonarian L. nigrum [29]. The absolute configuration of terpene 27 was determined to be 4S,5S,6R,7S,11S,12S by single crystal X-ray diffraction analysis with Cu Kα radiation [Flack parameter: 0.13(14)]. Sesquiterpene 26 exhibited a potent PTP1B inhibitory activity (IC50 10.67 μg/mL). It also showed moderate cytotoxic activities against the human tumor cell lines HT-29, Capan-1, and SNU-398 with IC50 values of 35.48, 42.55, and 25.17 μM, respectively. However, co-isolated metabolite 27 was inactive against PTP1B (IC50 > 20 μg/mL) or cell lines HT-29, Capan-1, and SNU-398 (all IC50 > 50 μM).
Recently, two members of this cluster, paralemnolin J (28) and (lS,8S,8aS)-l-[(E)-2′-acetoxy-l′-methylethenyl]-8,8a-dimethyl-3,4,6,7,8,8a-hexahydronaphthalen-2(1H)-one (29) (Figure 11), were isolated in the chemical investigation of a Balinese soft coral L. setoensis [16]. In terms of biological activity, cytotoxic effects against several solid tumor and leukemia cell lines HT-29, Capan-1, A549, and SNU-398 were assessed for compounds 28 and 29. As a result, both compounds showed weak cytotoxic activities against the test cell lines (all IC50 > 20 μM).
3.11. Nornardosinane Sesquiterpenes
Chemical study of Xisha alcyonarian L. nigrum afforded an uncommon nornardosinane sesquiterpene linardosinene A (30) [14] (Figure 12). The absolute configuration of 30 was determined by a modified Mosher’s method and TDDFT ECD approach. This isolate was evaluated for cytotoxicity against the THP-1 cell line and inhibitory activities against the PTP1B, BRD4, HDAC1, and HDAC6 protein kinases. However, it was inactive against the above-mentioned cell line and protein kinases.
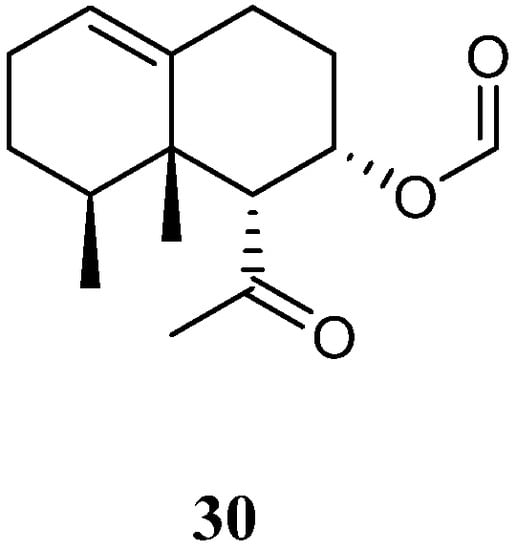
Figure 12. Chemical structure of the nornardosinane sesquiterpene from soft corals of the genus Litophyton.
3.12. Neolemnane Sesquiterpenes
A study on the chemical constituents of the Chinese soft coral L. nigrum yielded three new sesquiterpenes lineolemnenes A–C (31–33), which possessed the neolemnane carbon framework, together with the related known compound 4-acetoxy-2,8-neolemnadien-5-one (34) [14] (Figure 13). It might be worth pointing out that the absolute configuration of 34 was unambiguously determined to be 1S,4S,12S by X-ray diffraction analysis for the first time. The cytotoxicities of substances 31 and 32 against SNU-398, HT-29, Capan-1, and A549 were evaluated. This revealed that 31 and 32 only exhibited cytotoxic activity against SNU-398 with IC50 values of 44.4 and 27.6 μM, respectively, and none of them showed potent inhibitory activities against the PTP1B, BRD4, HDAC1, and HDAC6 protein kinases. Compound 34 was also found in the Indonesian soft coral L. setoensis, together with another sesquiterpene paralemnolin E (35) [16] (Figure 13). They were subjected to cytotoxic bioassays against several solid tumor and leukemia cell lines HT-29, Capan-1, A549, and SNU-398. The results revealed both two compounds had weak cytotoxic activities against the test cell lines (all IC50 > 20 μM). Parathyrsoidin E (36) (Figure 13) was reported in the soft coral Nephthea sp., which was collected from the Egyptian coasts of the Red Sea at Sharm El-Sheikh [39]. In silica study indicated this compound was a potential SARS-CoV-2 main protease inhibitor.
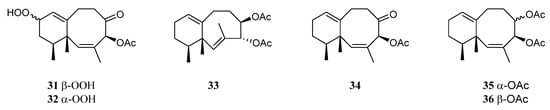
Figure 13. Chemical structures of the neolemnane sesquiterpenes from soft corals of the genus Litophyton.
3.13. Seconeolemnane Sesquiterpenes
A new sesquiterpene lineolemnene D (37) (Figure 14) was isolated and characterized from the Xisha soft coral L. nigrum [14]. Structurally, this compound possessed an unusual seconeolemnane skeleton. The absolute configuration of 37 was determined to be 1S,4R,12S by TDDFT ECD approach. Bioassays including cytotoxicity against the THP-1 cell line and inhibitory activities against the PTP1B, BRD4, HDAC1, and HDAC6 protein kinases were performed for this isolate. However, it was judged as inactive in these biotests.
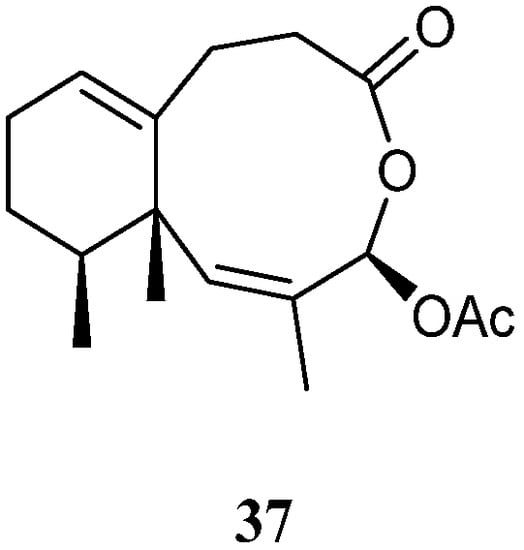
Figure 14. Chemical structure of the seconeolemnane sesquiterpene from soft corals of the genus Litophyton.
3.14. Kelsoane Sesquiterpenes
Interestingly, a new kelsoane-type sesquiterpene, namely kelsoenethiol (38) (Figure 15), was obtained from the Formosan soft coral N. erecta [28]. Its structure was elucidated with the assistance of quantum chemical calculations. The cytotoxicities of 38 against A-459, P-388, and HT-29 cancer cell lines were evaluated in vitro. The results revealed compound 38 exhibited cytotoxic activities against P-388 and HT-29 cells with ED50s of 1.3 and 1.8 μg/mL, respectively.
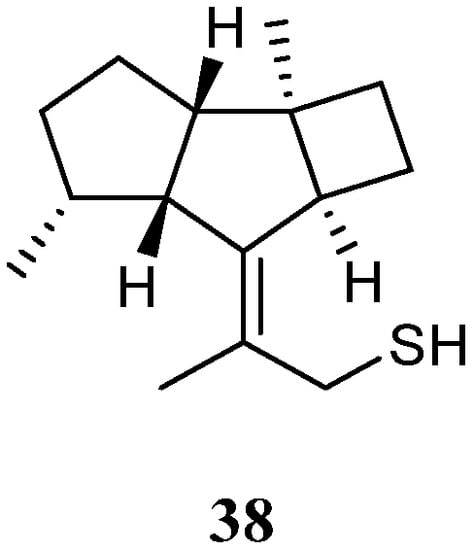
Figure 15. Chemical structure of the kelsoane sesquiterpene from soft corals of the genus Litophyton.
4. Bis-Sesquiterpenes
This group of terpenes were extremely uncommon secondary metabolites identified from the genus Litophyton with only two members. They were described as two subgroups according to their respective monomers: bis-kelsoane dimer and eremophilane-nardosinane dimer (Figure 16). All of them were the most unique type of natural products from this genus, since they were only obtained from the octocorals N. erecta and L. nigrum.
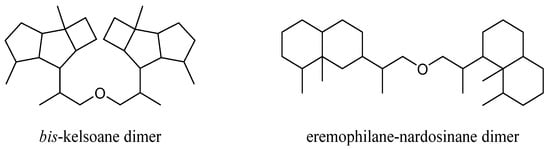
Figure 16. Carbon frameworks of the bis-sesquiterpenes from soft corals of the genus Litophyton.
5. Diterpenes
Diterpenes were the largest cluster of terpenes consisting of 46 compounds. Structurally, this category of secondary metabolites could be divided into six subgroups: cembranes, eunicellanes, serrulatanes, 5,9-cyclized serrulatanes, chabrolanes, and prenylbicyclogermacranes (Figure 17). Analysis of taxonomical distributions revealed they were obtained from L. viridis, L. arboreum, Litophyton viscudium, L. setoensis, Nephthea columnaris, Nephthea chablrolii, and unclearly indentified Litophyton sp. and Nephthea sp., which were collected in the Red Sea and the waters around Indonesia, Taiwan, Malaysia, and Japan.
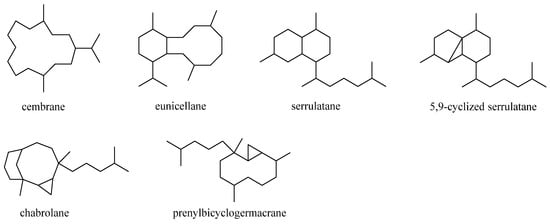
Figure 17. Carbon frameworks of the diterpenes reported from soft corals of the genus Litophyton.
This entry is adapted from the peer-reviewed paper 10.3390/md21100523
References
- Chakraborty, K.; Joy, M. High-value compounds from the molluscs of marine and estuarine ecosystems as prospective functional food ingredients: An overview. Food Res. Int. 2020, 137, 109637.
- Fonseca, S.; Amaral, M.N.; Reis, C.P.; Custódio, L. Marine natural products as innovative cosmetic ingredients. Mar. Drugs 2023, 21, 170.
- Banerjee, P.; Mandhare, A.; Bagalkote, V. Marine natural products as source of new drugs: An updated patent review (July 2018–July 2021). Expert Opin. Ther. Pat. 2022, 32, 317–363.
- Liu, M.; Zhang, X.; Li, G. Structural and biological insights into the hot-spot marine natural products reported from 2012 to 2021. Chin. J. Chem. 2022, 40, 1867–1889.
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2023, 40, 275–325.
- Chhetri, B.K.; Tedbury, P.R.; Sweeney-Jones, A.M.; Mani, L.; Soapi, K.; Manfredi, C.; Sorscher, E.; Sarafianos, S.G.; Kubanek, J. Marine natural products as leads against SARS-CoV-2 infection. J. Nat. Prod. 2022, 85, 657–665.
- El-Desoky, A.H.H.; Tsukamoto, S. Marine natural products that inhibit osteoclastogenesis and promote osteoblast differentiation. J. Nat. Med. 2022, 76, 575–583.
- Deng, Y.; Liu, Y.; Li, J.; Wang, X.; He, S.; Yan, X.; Shi, Y.; Zhang, W.; Ding, L. Marine natural products and their synthetic analogs as promising antibiofilm agents for antibiotics discovery and development. Eur. J. Med. Chem. 2022, 239, 114513.
- Ren, X.; Xie, X.; Chen, B.; Liu, L.; Jiang, C.; Qian, Q. Marine natural products: A potential source of anti-hepatocellular carcinoma drugs. J. Med. Chem. 2021, 64, 7879–7899.
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine natural products in clinical use. Mar. Drugs 2022, 20, 528.
- van Ofwegen, L.P. The genus Litophyton Forskål, 1775 (Octocorallia, Alcyonacea, Nephtheidae) in the Red Sea and the western Indian Ocean. Zookeys 2016, 567, 1–128.
- van Ofwegen, L.; Groenenberg, D.S.J. A centuries old problem in nephtheid taxonomy approached using DNA data (Coelenterata: Alcyonacea). Contrib. Zool. 2007, 76, 153–178.
- World List of Octocorallia. Litophyton Forskål, 1775. World Register of Marine Species. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=204523 (accessed on 28 August 2023).
- Yang, F.; Li, S.-W.; Zhang, J.; Liang, L.-F.; Lu, Y.-H.; Guo, Y.-W. Uncommon nornardosinane, seconeolemnane and related sesquiterpenoids from Xisha soft coral Litophyton nigrum. Bioorg. Chem. 2020, 96, 103636.
- van Ofwegen, L.P. The genus Litophyton Forskål, 1775 (Octocorallia: Alcyonacea: Nephtheidae) from Australia. Zootaxa 2020, 4764, 1–131.
- Li, S.-W.; Mudianta, I.W.; Cuadrado, C.; Li, G.; Yudasmara, G.A.; Setiabudi, G.I.; Daranas, A.H.; Guo, Y.-W. Litosetoenins A–E, diterpenoids from the soft coral Litophyton setoensis, backbone-rearranged through divergent cyclization achieved by epoxide reactivity inversion. J. Org. Chem. 2021, 86, 11771–11781.
- Iwagawa, T.; Kusatsu, D.; Tsuha, K.; Hamada, T.; Okamura, H.; Furukawa, T.; Akiyama, S.-I.; Doe, M.; Morimoto, Y.; Iwase, F.; et al. Cytotoxic eunicellin-type diterpenes from the soft coral Litophyton viscudium. Heterocycles 2011, 83, 2149–2155, Erratum in Heterocycles 2012, 85, 2615–2615.
- Tursch, B. Chemical protection of a fish (Abudefduf leucogaster Bleeker) by a soft coral (Litophyton viridis May). J. Chem. Ecol. 1982, 8, 1421–1428.
- Ashry, M.; Askar, H.; Alian, A.; Zidan, S.A.H.; El-Sahra, D.G.; Abdel-Wahhab, K.G.; Lamlom, S.F.; Abdelsalam, N.R.; Abd El-Hack, M.E.; Gomaa, H.F. The antioxidant and antitumor efficiency of Litophyton sp. extract in DMH-induced colon cancer in male rats. Life 2022, 12, 1470.
- Hawas, U.W.; Abou El-Kassem, L.T.; Fahmy, M.A.; Farghaly, A.A.; Hassan, Z.M. A new pseudoguaiane-type sesquiterpene and potential genotoxicity and antigenotoxicity effect of the soft coral Litophyton arboreum extract. Lett. Org. Chem. 2018, 15, 1060–1064.
- Ellithey, M.S.; Lall, N.; Hussein, A.A.; Meyer, D. Cytotoxic and HIV-1 enzyme inhibitory activities of Red Sea marine organisms. BMC Complem. Altern. Med. 2014, 14, 77.
- Abdel-Tawab, A.M.; Fayad, W.; Shreadah, M.A.; Nassar, M.I.; Abou-Elzaha, M.M.; Abdel-Mogib, M. GC/MS identification and biological evaluation of the Red Sea soft coral Nephthea molle extracts. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 595–602.
- Tanod, W.A.; Yanuhar, U.; Maftuch; Putra, M.Y.; Risjani, Y. Screening of NO inhibitor release activity from soft coral extracts origin Palu Bay, Central Sulawesi, Indonesia. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2019, 18, 126–141.
- Hassan, N.H.; El-Hawary, S.S.; Emam, M.; Rabeh, M.A.; Abdelmohsen, U.R.; Selim, N.M. Potential inhibitors of CYP51 enzyme in dermatophytes by Red Sea soft coral Nephthea sp.: In silico and molecular networking studies. ACS Omega 2022, 7, 13808–13817.
- Hassan, N.H.; El-Hawary, S.S.; Emam, M.; Rabeh, M.A.; Tantawy, M.A.; Seif, M.; Abd-Elal, R.M.A.; Bringmann, G.; Abdelmohsen, U.R.; Selim, N.M. Pectin nanoparticle-loaded soft coral Nephthea sp. extract as in situ gel enhances chronic wound healing: In vitro, in vivo, and in silico studies. Pharmaceuticals 2023, 16, 957.
- Schmitz, F.J.; Vanderah, D.J.; Ciereszko, L.S. Marine natural products: Nephthenol and epoxynephthenol acetate, cembrene derivatives from a soft coral. J. Chem. Soc. Chem. Commun. 1974, 5, 407–408.
- Tursch, B.; Braekman, J.C.; Daloze, D. Chemical studies of marine invertebrates—XIII 2-Hydroxynephtenol, a novel cembrane diterpene from the soft coral Litophyton viridis (Coelenterata, Octocorallia, Alcyonacea). Bull. Soc. Chim. Belg. 1975, 84, 767–774.
- Cheng, S.-Y.; Shih, N.-L.; Hou, K.-Y.; Ger, M.-J.; Yang, C.-N.; Wang, S.-K.; Duh, C.-Y. Kelsoenethiol and dikelsoenyl ether, two unique kelsoane-type sesquiterpenes, from the Formosan soft coral Nephthea erecta. Bioorg. Med. Chem. Lett. 2014, 24, 473–475.
- Yang, F.; Hua, Q.; Yao, L.-G.; Liang, L.-F.; Lou, Y.-X.; Lu, Y.-H.; An, F.-L.; Guo, Y.-W. One uncommon bis-sesquiterpenoid from Xisha soft coral Litophyton nigrum. Tetrahedron Lett. 2022, 88, 153571.
- Grote, D.; Dahse, H.-M.; Seifert, K. Furanocembranoids from the soft corals Sinularia asterolobata and Litophyton arboreum. Chem. Biodivers. 2008, 5, 2449–2456.
- Ahmed, M.M.A.; Ragab, E.A.; Zayed, A.; El-Ghaly, E.M.; Ismail, S.K.; Khan, S.I.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Litoarbolide A: An undescribed sesquiterpenoid from the Red Sea soft coral Litophyton arboreum with an in vitro anti-malarial activity evaluation. Nat. Prod. Res. 2023, 37, 542–550.
- Ellithey, M.S.; Lall, N.; Hussein, A.A.; Meyer, D. Cytotoxic, cytostatic and HIV-1 PR inhibitory activities of the soft coral Litophyton arboreum. Mar. Drugs 2013, 11, 4917–4936.
- Ellithey, M.S.; Ahmed, H.H. Bioactive marine-derived compounds as potential anticancer candidates. Asian J. Pharm. Clin. Res. 2018, 11, 464–466.
- Abou El-Kassem, L.T.; Hawas, U.W.; El-Desouky, S.K.; Al-Farawati, R. Sesquiterpenes from the Saudi Red Sea: Litophyton arboreum with their cytotoxic and antimicrobial activities. Z. Naturforsch. C-J. Biosci. 2018, 73, 9–14.
- Mahmoud, A.H.; Zidan, S.A.H.; Samy, M.N.; Alian, A.; Abdelmohsen, U.R.; Fouad, M.A.; Kamel, M.S.; Matsunami, K. Cytotoxicity and chemical profiling of the Red Sea soft corals Litophyton arboreum. Nat. Prod. Res. 2022, 36, 4261–4265.
- Hegazy, M.-E.F.; Gamal-Eldeen, A.M.; Mohamed, T.A.; Alhammady, M.A.; Hassanien, A.A.; Shreadah, M.A.; Abdelgawad, I.I.; Elkady, E.M.; Paré, P.W. New cytotoxic constituents from the Red Sea soft coral Nephthea sp. Nat. Prod. Res. 2016, 30, 1266–1272.
- Ghandourah, M.A.; Alarif, W.M.; Abdel-Lateff, A.; Al-Lihaibi, S.S.; Ayyad, S.-E.N.; Basaif, S.A.; Badria, F.A. Two new terpenoidal derivatives: A himachalene-type sesquiterpene and 13,14-secosteroid from the soft coral Litophyton arboreum. Med. Chem. Res. 2015, 24, 4070–4077.
- Yang, F.; Hua, Q.; Yao, L.-G.; Liang, L.-F.; Lu, Y.-H.; An, F.-L.; Guo, Y.-W. Further new nardosinane-type sesquiterpenoids from the Xisha soft coral Litophyton nigrum. Fitoterapia 2021, 151, 104906.
- Abdelhafez, O.H.; Fahim, J.R.; Mustafa, M.; AboulMagd, A.M.; Desoukey, S.Y.; Hayallah, A.M.; Kamel, M.S.; Abdelmohsen, U.R. Natural metabolites from the soft coral Nephthea sp. as potential SARS-CoV-2 main protease inhibitors. Nat. Prod. Res. 2022, 36, 2893–2896.
This entry is offline, you can click here to edit this entry!
