2. Omes, Omics and Multi-Omics
Essentially all diseases, even those not formally pertaining to the category of “complex diseases”, are multifaceted because of the huge amount of varied interconnected biological components and events involved in their pathophysiology [
7]. With the appreciation of the innumerable components of any disease came the realization that the study of any single component in isolation would never solve the intricacies of the underlying biology. From the above realization emerged the need to study all components in a far more comprehensive way, hence the emphasis on “omes” and “omics”, defined, respectively, as the totality of any particular field and their study. Although the analysis of single omics can provide relevant information, the integration of several omics, i.e., “multi-omics”, allows a much more comprehensive view of the mechanisms underlying any disease [
8,
9]. All of the omes believe to be involved in inflammatory bowel disease (IBD) are highly complex and variable [
10,
11], and the investigation of any ome alone can barely open a narrow window into the intricate mechanisms underlying ulcerative colitis (UC) or Crohn’s disease (CD). This is as well exemplified by the numerous but insular genomics studies of IBD, where early and subsequent massive DNA sequencing analysis from several thousand patients identified hundreds of common and rare genetic variants associated with CD or UC without generating clear-cut or definitive clues to their etiology, pathogenesis, classification, diagnosis or therapy [
12,
13,
14]. In fact, no clinically useful IBD genetic markers have been identified so far, not even for the variants of
NOD2 that are closely associated with ileal CD at the clinical level without influencing the response to therapy [
15,
16,
17]. After a plethora of reports on the genetics of IBD, there is still no evidence that assessing genetic factors independently of other contributing factors can provide a practical guide for diagnosis or therapy of CD or UC [
17]. Given this evidence, and the vast amount of literature available to the reader, the topic of genomics will not be specifically addressed in this review. However, to gain a truly comprehensive understanding of IBD, it is still mandatory to include the genome in combination with other omes, hence the need for “multi-omics” studies carried out with integrative computational methodologies [
18].
3. Multi-Omics in Health
While the complexity of any disease status is an accepted reality, it is often forgotten that health is also an extremely complex condition [
19]. The maintenance of health, with all the endless challenges imposed by growth, environmental factors, adaptation, nutrition, metabolism, immunity, behavior, and mental and physical demands, requires a perfect coordination of innumerous physiological functions and their respective omes [
9]. However, the study of health multi-omics lags far behind that of disease multi-omics, hindering and delaying a better understanding of pathophysiological events resulting from the derangements of healthy omes [
20,
21,
22].
The advent of multi-omics is connected to and strictly dependent on new technologies that allow a detailed molecular analysis of omes [
23]. The best example is the use of next-generation sequencing (NGS) of DNA with the purpose of defining the complete human genome with its many variants [
24]. Sequencing platforms are now used to dissect the epigenome, transcriptome and metagenome, while mass spectroscopy platforms are used to study the proteome and the metabolome [
25]. However, it is essential that all omes relevant to both health and disease are studied in a coordinated and integrated way, a fundamental point that will be discussed in greater detail later on in this review.
4. Multi-Omics in Complex Diseases
The field of oncology has pioneered most of the omics technologies used for the investigation of complex diseases. Starting in 2006, The Cancer Genome Atlas (TCGA) research network, with the participation of investigators worldwide, has profiled 33 different types of human tumors and discovered numerous molecular abnormalities at the DNA, RNA, protein and epigenetic level associated with distinct cancer types [
26]. This landmark approach launched what has now become the conventional approach to studying multiple non-cancerous complex diseases in various human organs and systems. Regrettably, progress with these autoimmune or chronic inflammatory diseases has been much slower than with cancer due to lack of universal coordination and collaboration and more limited resources. Nonetheless, numerous reports of omics or multi-omics studies have been published for a variety of diseases, like rheumatoid arthritis, systemic lupus erythematosus, asthma, chronic obstructive pulmonary disease, and even irritable bowel syndrome and autism [
27,
28,
29,
30,
31,
32]. It should be kept in mind that these complex diseases occur in vastly different organs and tissues that have their own tissue-specific regulatory circuits in both health and disease, and these differences will be reflected in the respective multi-omics analyses [
33].
5. Multi-Omics in IBD
The importance of omics in intestinal inflammation and their potential for advancing disease understanding, biomarker discovery and new therapies have been recognized since the mid-2000s [
34], but the appreciation of omics by the medical community has been sluggish and only recently have studies begun to be undertaken and generate some tangible results. Though not to the same degree of the TCGA network, efforts to create working groups for the global study of omics and multi-omics in IBD have also been made, like the 1000IBD project (
https://1000ibd.org, accessed on 10 August 2023) [
35]. The project recruits and prospectively follows adult patients with new or existing IBD of different phenotypes and collects information about several omes (exposome, genome, transcriptome and metagenome, drug response, etc.). The 1000IBD project has generated a number of publications on various topics, like the modulation of intestinal gene expression by inflammation [
36], the association of dietary patterns with selective features of the gut microbiota [
37], the impact of drugs on gut microbiota composition and function [
38], the identification of environmental factors linked to IBD development [
39], the association of IBD genotypes and phenotypes with the plasma proteome [
40], as well as reviews on multi-omic data availability in IBD [
41], and several other aspects of IBD pathophysiology. Another similar but more recent group initiative is the European ImmUniverse Consortium (
https://www.imi.europa.eu/projects-results/project-factsheets/immuniverse, accessed on 8 January 2019) which was formed to create a multi-omics integrative approach to personalized medicine in immune-mediated inflammatory diseases, including IBD [
42]. Several other IBD omics-oriented initiatives are also under way worldwide [
43].
However, the vast majority of reports on IBD multi-omics are still the result of work carried out by individual investigators and, whether as reviews or original reports, have substantially expanded UC and CD omics information on a wide variety of topics. Reviews tend to emphasize the importance of multi-omics in IBD for the goal of delivering more effective treatment [
44] or precision medicine to UC and CD patients [
45,
46], or the potential of multi-omics to identify predictive biomarkers for the discovery of IBD risk factors [
47], while other reviews call attention to the numerous challenges of designing and analyzing multi-omics IBD data [
48].
The number of omics reported in the literature at large or listed in formal databases is large (Table 1). However, what omics are relevant to IBD, to what degree, which ones should be prioritized for study, which ones should be functionally integrated, and which omes research should be our main focus is a critical and unresolved issue.
Table 1. Omes and omics glossary.
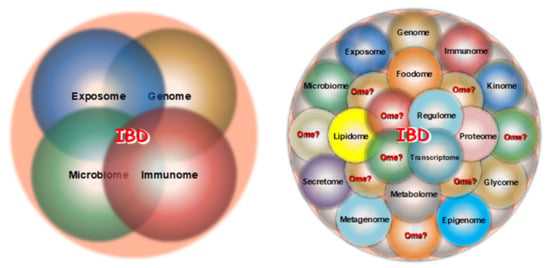
Until recently, four major omes received the bulk of the attention from IBD investigators: the exposome, the genome, the microbiome and the immunome (Figure 1, left panel). With the recent advent of the sequencing and mass spectroscopy platform, the type and number of omes accessible to IBD-related investigation have drastically increased, including the epigenome, transcriptome, proteome, metabolome, etc., but many additional unexplored omes are undoubtedly relevant to CD and UC pathophysiology (Figure 1, right panel).
Figure 1. Traditional (left panel) and more recent (right panel) omes under investigation in IBD.
5.1. Exposomics
The study of the environmental factors (the exposome) as they related to IBD overall, or IBD pathogenesis in particular, is the most difficult and problematic because of the endless number of factors involved, their multiplicity of actions, the constant generation of new man-made substances that permeate the environment at large and modify the exposome, the individuality of the exposure process by humans and the unique response of each person [
49,
50,
51]. Reports and reviews on the assumed or demonstrable impact of environmental risk factors in IBD are plentiful [
39,
52,
53], but it is logistically and practically impossible to identify all relevant factors in each IBD patient and derive usable omics data to be factored in for multi-omics integration.
5.2. Microbiomics
The importance of the gut microbiome to IBD pathogenesis has been long established [
54] and progressively explored in greater depth with the adoption of metagenomic analyses [
55]. Studies analyzing the composition and function of the gut microbiota in UC or CD patients are numerous, but when performed in isolation they have generated unclear and inconsistent results [
56]. However, metagenomic analyses of the gut microbiome combined with other omes can result in more relevant information about the role of microbes in the pathobiology of IBD. For instance, a study performed as part of the Human Functional Genomics Project explored the combination and functional relationship of multi-omic microbial and cytokine profiles and found a link between the human gut microbiome and inflammatory cytokine production [
57]. Using longitudinally collected blood, stools and biopsy samples, an IBD multi-omics (metagenome, metatranscriptome, proteome, metabolome, and virome) database has been created, revealing that patient disease activity is marked by temporal increases and shifts in taxonomy, as well as functional and biochemical composition [
58]. The combination of metagenomics, metatranscriptomics and metaproteomics can uncover multiple microbial bioactive molecules likely to interact with the host immune system in IBD, and inform us about the broad and dynamic host–microbial interactions involved in IBD pathogenesis [
59]. While enlightening and exciting, all results of microbiomics reports must be interpreted with caution in view of the extensive impact of genetics [
60], lifestyle [
61], nutrition [
62], immunity [
63], common drugs or drugs used to treat IBD patients [
38,
64] on gut microbiota composition and function.
5.3. Immunomics
Immunology has dominated the study of IBD for a long time, and only in the last decade or so has its dominance partially diminished due to a growing interest in the gut microbiota and other non-immune factors in IBD pathogenesis [
65]. The key role of the immune response in IBD is unquestionable, but this response is influenced by a myriad of factors such as the host, environment, genes, microbes, nutrition, behavior, season of the year, stress, etc. [
51,
57,
63,
66,
67]. This multiplicity of unpredictable and uncontrollable factors puts constant demands on the immune system, which responds by continuously adapting in a vigorous way, leading to both beneficial and harmful effects [
68]. This multidimensional response makes it unrealistic to expect that the immune system alone can provide reproducible and reliable markers or omics that define specific biological states in multifactorial diseases like CD or UC. A good example is a report which claimed that the transcriptional signature of circulating CD8+ T cells could predict the disease course of patients with CD and UC [
69], a claim that could not be reproduced in a subsequent study [
70]. These contrasting results are likely be due to the plasticity of CD8+ T cells in IBD [
71], a quality inherent to all immune cells residing in specific tissue microenvironments [
72], which makes them and their products, like cytokines, essentially impossible to use as predictable and reproducible qualitative or quantitative measurements of any immune response [
73]. Fundamental abnormalities of the immune response are clearly involved in the pathogenesis of UC and CD and, considering the variability and unpredictability of the immune response, such abnormalities cannot be evaluated in isolation, but integrated with multiple other omes under the umbrella of the modern comprehensive notion of the IBD interactome [
74].
5.4. Epigenomics
The field of epigenetics, variably defined as DNA-independent changes in gene expression or the transgenerational effects and/or inherited expression states [
75], is a more recently and relatively less investigated omic in IBD [
76], but it is of crucial importance because of the link it establishes between genes and the exposome [
77]. Intestinal biopsies obtained from pediatric CD and UC patients submitted to combined DNA methylation and transcription analyses were able to identify disease subtypes and an association with clinical outcome [
78]. A study by Kalla et al. performed multi-omic integration of the methylome, genome and transcriptome measured in the peripheral blood of UC and CD patients and identified multiple differentially methylated positions in IBD with an association with treatment escalation or need for surgery [
79]. The latter group of investigators recently reported a differential and variable methylation in CD patients developing clinical recurrence after surgery [
80]. The study of epigenetics in IBD is now attracting more and more attention, but it also must be considered in the context of interactions with the genome and the exposome [
81].
5.5. Proteomics, Metabolomics, Lipidomics
The possibility of using serum proteomics for biomarker discovery in IBD has been considered for a long time [
82], and this approach, particularly when combined with metabolomics and lipidomics, could help us to discover complementary biomarkers, monitor and predict response to therapy and promote personalized medicine [
83,
84]. The combined alterations in serum lipids, amino acids and energy metabolites have been reported to distinguish UC from CD and healthy controls [
85], while a study by Fan et al. compared plasma lipid profiles in UC vs. CD patients and found that a number of ether lipids were negatively associated with CD [
86]. Another study reported that neither metagenomic or host genetics could distinguish ileal and colonic CD, but this could be accomplished via metabolomics and metaproteomics analyses using mass spectroscopy [
87].
5.6. Single-Cell Technologies, Omics, Multi-Omics and Spatial Multi-Omics
With the progress prompted by the development of many new cellular and molecular tools complemented by the even more rapid deployment of artificial intelligence, machine and deep learning analytical methods, the possibility of studying omics and multiomics at the single-cell level has become an exciting reality [
88]. An enormous amount of literature has rapidly accumulated on single cell omics, multi-omics and spatial transcriptomics in different cells and organs. Initial studies focused primarily on single-cell transcriptomics [
89,
90], resulting in the universal and fundamental discovery of an unsuspected extreme degree of cell heterogeneity of both immune [
91,
92] and non-immune cells [
93,
94] in essentially all tissues, including the gastrointestinal tract [
95,
96,
97]. These reports were then followed by single-cell multi-omics studies in which the genome, epigenome, transcriptome, proteome, or other omes could be assessed in the same cells [
88] and the subsequent investigation of single-cell multi-omics, i.e., the detection and topographical mapping of multiple omes in specific tissue types [
98] in both normal and inflamed tissues [
99]. The field of single cell analysis, omics and multi-omics is still evolving very rapidly and in the near future, reference maps of the whole human body will become available with an unprecedented level of cellular and molecular precision [
100].
In the field of gastroenterology and IBD in particular, similar studies have emerged that also show vast single cell heterogeneity in immune and epithelial cells in both UC and CD [
101,
102,
103]. Recently, additional studies have appeared describing fibroblast heterogeneity and cellular interactions of a full thickness single-cell transcriptomic atlas of the strictured CD intestine [
104], and the single-cell spatial proteomics and transcriptomics analysis of colonic biopsies of patients with UC receiving treatment with vedolizumab [
105].
6. Clinical Applications of Multi-Omics Analyses
The primary goal behind the study of multi-omics in IBD is the discovery of novel tools that afford more precise diagnostic and management means at the bedside. Based on this very desirable goal, a large and growing number of studies have been and are still being published which claim that the utilization of multi-omics and their different combinations can help with the discovery of basic, translational, and clinical elements of UC and CD. The execution of these studies is justifiable and the results are of interest. However, most of the claims so far made must be carefully assessed and interpreted in the light of more stringent criteria, as discussed later under the subtitle Pitfalls and Limitations of Current IBD Multi-omics Studies.
6.1. Biomarker Identification
The quest to identify disease biomarkers is as old as the field of medicine. The popular biomarkers commonly used for the evaluation of UC and CD patents, like C reactive protein and fecal calprotectin, are not true disease biomarkers as they simply reflect the process of inflammation and its degree, but are not specific and their levels increase as a result of many other conditions [
106]. The same can be said for serological biomarkers, whose combined evaluation is of uncertain value for the prediction of disease progression or response to treatment [
107]. In 2016, the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) created the FDA-NIH Biomarker Working Group with the goal of better defining what is intended for “biomarker” and their many types; this was last updated in 2021 [
108]. The FDA-NIH working group identified the following types of biomarkers: susceptibility/risk, predictive, diagnostic, monitoring, pharmacodynamic/response, safety, prognostic as well as “reasonably likely surrogate endpoints” [
108]. In principle, all of them are of interest in IBD and the topic has generated a great deal of attention and a huge volume of publications [
109]. The possibility of biomarker discovery using omics and multi-omics approaches has been a goal for multiple inflammatory and neoplastic conditions [
110,
111,
112,
113,
114], including IBD [
115], but so far precise and definitive biomarkers have yet to be found.
6.2. Prediction of Remission and Relapse
The identification of biomarkers that predict clinical relapse or clinical remission of IBD patients using multi-omics profiling has been the target of numerous studies. One study based on profiling of the blood proteome, metabolome and microbiome claimed that a proinflammatory state predisposing to clinical relapse could be identified in UC and CD patients [
116], while another study carried out tissue and blood plasma proteome and plasma metabolomics profiling of UC patients and concluded that biomarkers indicative of functional remission could be identified [
117]. A very recent report found that certain blood protein profiles were associated with the risk of clinical relapse in patients who stopped infliximab therapy [
118], a potentially useful solution to the vexing issue of when to stop this common form of biological therapy. None of these studies have been replicated or validated until now and their value remains unclear.
6.3. Response to Therapy
In an early pilot study, serum proteomics were used to establish a model to predict response to infliximab treatment, with the results suggesting that response was associated with platelet metabolism [
119]. A subsequent study based on prospectively collected blood and stool samples carried out metagenomic, serum metabolomic and serum proteomic measurements and found that some microbial factors affected the response of IBD patients receiving biological therapies [
120]. A more recent study by Mishra et al. performed a longitudinal, blood-based multi-omics (RNAseq and genome-wide DNA methylation) study in two prospective IBD cohorts given TNF antagonists to predict therapy response, but no consistent predictive molecular signatures could be found [
121]. Thus, the prediction of response to therapy in IBD patients based on results of multi-omics analyses appears to be at a preliminary stage and the results vary depending on the number and combination of the omics included in the prediction modeling. Perhaps more importantly, it still remains to be verified whether any of the newly described omics-based biomarkers are indeed better than traditional ones, like C-reactive protein [
122].
6.4. Precision Medicine
The implementation of precision medicine is the ultimate goal behind the study of multi-omics in complex diseases [
123]. Precision medicine has many definitions, the one officially released by the NIH being one of the most comprehensive and commonly cited: “
An emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person” (
www.nih.gov/precisionmedicine, accessed on 10 August 2023). The need for and importance of developing precision medicine are undeniable and have an enormous potential, but they require the integration of clinical data with multiple molecular profiles, and this is only possible with the most advanced computational methods and a close collaboration of clinicians, researchers and bioinformaticians [
124]. With the advent of NGS and various other technologies that allow molecular characterization of health and disease, the quest for precision medicine in IBD has also been relying on omics and multi-omics studies for its fulfilment. This goal is extremely challenging and has yet to be achieved [
125], but various publications reinforce the fundamental importance of pursuing multi-omics studies [
126,
127], including a recent series of state-of-the-art reports by the European Crohn’s & Colitis Organization (ECCO) [
11,
128,
129,
130].