Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
In the field of oncology, the Signaling Lymphocyte Activation Molecule (SLAM) family is emerging as pivotal in modulating immune responses within tumor environments. The SLAM family comprises nine receptors, mainly found on immune cell surfaces. These receptors play complex roles in the interaction between cancer and the host immune system. Research suggests SLAM’s role in both enhancing and dampening tumor-immune responses, influencing the progression and treatment outcomes of various cancers.
- SLAM
- SLAMF
- cancer
- immune modulation
- CLL
- AML
1. Chronic Lymphocytic Leukemia (CLL)
Chronic Lymphocytic Leukemia, predominantly observed in European and North American adult populations, manifests as a marked proliferation of mature B-lymphocytes within the bone marrow, lymph nodes, and bloodstream [25]. The SLAM receptors have gained prominence within this milieu due to their pivotal role in modulating the immune system, especially in the context of CLL.
One such notable receptor, SLAMF1, serves as a suppressor of IL-10 expression and release on the B-CLL cell surface. There is a documented correlation between SLAMF1’s presence and improved clinical prognosis in CLL patients [26]. Notably, an increased expression of both SLAMF1 and SLAMF7 emerges as a positive prognostic sign. Such patients, without the diminished activity of these receptors, may display heightened NK cell-induced cytotoxicity, indicating a more robust immunological oversight. On the other hand, diminished SLAMF1 expression is viewed with apprehension, as it may influence the timing of treatment initiation, as well as overall patient survival rates. Delving deeper into the mechanism, there is a hypothesis suggesting that SLAMF1 loss in CLL could perturb genetic routes that control chemotaxis, autophagy, and responses to treatment [27,28].
A significant interplay to consider in CLL’s pathobiology is between SLAMF1 and the CD180 receptor pathway. This dynamic interaction selectively inhibits both Akt and MAPK signaling. Intriguingly, both SLAMF1 and CD180 have been spotlighted as enhancers of CD20 expression. This might insinuate that B-CLL cells with these receptors are potentially more susceptible to immunotherapies targeting CD20 [29]. These findings suggest that the expression patterns of SLAM receptors can serve as potential biomarkers for predicting disease progression and patient outcomes.
In terms of chemotherapeutic susceptibility, although direct associations between SLAMF1 levels and drug sensitivity in B-CLL were elusive, there is documentation indicating that an elevated mRNA expression of the mSLAMF1 isoform correlates with fludarabine sensitivity and cyclophosphamide resistance. Conversely, an upsurge in nSLAMF1 mRNA expression could signify resistance to fludarabine [29]. On a hopeful note for combination treatments, preliminary research posits that synchronizing an anti-SLAMF6 antibody with the drug ibrutinib effectively stymies CLL cell growth, paving the way for potential CLL therapeutic avenues [30].
2. Acute Myeloid Leukemia (AML)
AML is a rapidly progressing cancer of hematopoietic progenitor cells, leading to the unregulated growth of immature myeloid cells. Although survival rates have improved for chronic myeloid and lymphocytic leukemias, the prognosis for adult AML remains relatively unchanged over the years [31,32]. Within the AML landscape, SLAMF2 is recognized as a positive prognostic indicator. However, it is frequently found to be down-regulated in affected patients. This reduction in SLAMF2 levels might be a tactic employed by AML to avoid detection and elimination by Natural Killer (NK) cells. Intriguingly, methylation is one of the regulatory mechanisms affecting SLAMF2 expression. Studies suggest that hypomethylating agents could boost SLAMF2 levels, potentially intensifying NK cell-mediated attack against AML cells in vitro [33]. Delving deeper into the molecular underpinnings of AML, research indicates the role of the fusion gene AML1-ETO. It is postulated that this gene facilitates the immune evasion of AML cells by specifically targeting CD48 (SLAMF2) [33]. Corroborating this, another study revealed the ability of AML cells to dampen the immune response by epigenetically down-regulating CD48 [34].
3. Multiple Myeloma (MM)
Multiple myeloma (MM) is a hematologic malignancy marked by excessive growth of clonal plasma cells within the bone marrow. This rapid proliferation leads to severe complications such as bone degradation, renal problems, anemia, and hypercalcemia. Annually, an estimated 34,920 individuals in the U.S. and a staggering 588,161 worldwide receive an MM diagnosis [35].
SLAMF3 stands out as a consistent marker on MM cells, regardless of the disease’s phase. Attenuating or eradicating SLAMF3 has a dual advantage: it not only restricts MM cell growth but also makes them more susceptible to drug-triggered apoptosis. A surge in serum-soluble SLAMF3 concentrations could act as an indicative marker for MM’s evolution. Interventions targeting SLAMF3 might offer a way to target the therapy-resistant cells that persist in the bone marrow after standard treatments [36]. Precisely, SLAMF3’s engagement in MM cells activates pathways like ERK signaling and certain transcription factors, supporting cellular survival, growth, movement, differentiation, and resistance against apoptosis. The utilization of anti-SLAMF3 antibodies could hold back MM progression [37].
Pioneering studies have crafted SLAMF3-based CAR T cells, showcasing remarkable efficacy against various MM cells both in lab settings and animal models [38]. Another member of the same family, SLAMF5, is also prevalent in MM cells. Cells release the macrophage migration inhibitory factor (MIF), stimulating its expression and leading to Myeloid-Derived Suppressor Cells (MDSCs) aggregation. This consequently up-regulates PD-L1 expression in these cells. Targeting SLAMF5 can counteract this MDSCs buildup, resulting in enhanced T cell activation and a reduced tumor presence [34,39].
Elotuzumab, a specialized humanized IgG1 monoclonal antibody aimed at SLAMF7, has gained approval for use in MM patients. It is prescribed alongside lenalidomide and dexamethasone when 1–3 treatment regimens have been ineffective. Alternatively, it is combined with pomalidomide and dexamethasone after two unsuccessful treatments, specifically those involving lenalidomide and a protease inhibitor [40]. Elotuzumab functions as an immune activator, disconnecting MM cells from the bone marrow’s stromal cells and initiating ADCC against the MM cells [9,41]. Significantly, Elotuzumab amplifies the bond between NK cells and MM cells, driving the NK cells to target and destroy MM cells more extensively than through the typical ADCC process [42,43] (Figure 1).
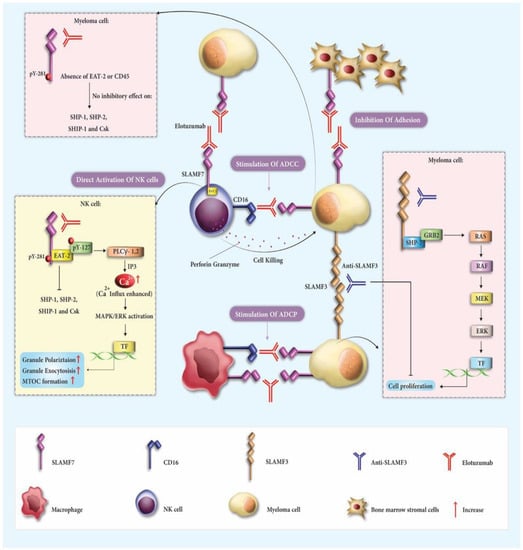
Figure 1. The therapeutic mechanisms involving elotuzumab and Anti-SLAMF3 in multiple myeloma (MM). Elotuzumab engages with various receptors and pathways to coordinate an immune response. It targets signaling lymphocytic activation molecule family receptor (SLAMF7) on both natural killer (NK) cells and MM cells, activating NK cells through EAT-2 and inducing antibody-dependent cellular cytotoxicity (ADCC) via Fc component interaction with CD16. The antibody also disrupts the homotypic interactions between SLAMF7 on MM cells and bone marrow stromal cells (BMSCs), inhibiting adhesion. The signaling cascade initiated by elotuzumab enhances cytotoxicity through the ERK pathway, polarizes cytolytic granules, and enables macrophage-mediated antibody-dependent cellular phagocytosis (ADCP). In the context of MM, SLAMF3 molecules also play a role by forming connections that trigger the ERK signaling pathway and activate distinct transcription factors. The potential for limiting malignancy growth exists through antibodies targeting SLAMF3. Collectively, these mechanisms activate innate immune responses against MM cells and enhance the therapeutic efficacy of both elotuzumab and Anti-SLAMF3.
Toxicities from Elotuzumab include infusion reactions experienced by 10% of patients, with symptoms such as fever, chills, and hypertension. A significant 81.4% of patients reported infections, including opportunistic and fungal types. Additionally, 9.1% of patients developed another invasive malignancy, termed Second Primary Malignancies. Hepatotoxicity was evident in 2.5% of patients, indicated by elevated liver enzymes. Elotuzumab can also interfere with the determination of a complete response in certain myeloma patients. Other commonly reported adverse reactions are fatigue, diarrhea, pyrexia, constipation, cough, peripheral neuropathy, nasopharyngitis, respiratory infections, decreased appetite, and pneumonia [40].
A commendable effort by Bristol-Myers Squibb to provide Expanded Access to Elotuzumab for Multiple Myeloma patients in several countries was initiated and intended for patients who were running out of treatment options [44]. An interesting trial from Tulane University School of Medicine sought to combine Elotuzumab with Selinexor and dexamethasone for relapsed refractory multiple myeloma (RRMM) patients resistant to several treatment lines. However, the study was withdrawn due to a lack of funding from the collaborating pharmaceutical company [45]. It is worth noting that Elotuzumab has the potential to induce ADCC even in MM cells that do not respond to treatments like bortezomib. Its binding mechanism with SLAMF7 directly activates NK cells and, concurrently, prevents MM cell attachment to bone marrow stromal cells due to inherent SLAMF7 interactions [46].
4. Head and Neck Squamous Cell Carcinoma (HNSCC)
HNSCC, the predominant head and neck tumor histology, has a global incidence nearing 890,000 cases each year. It originates from the mucosa of the upper aerodigestive tract, encompassing areas like the oral cavity, nasopharynx, pharynx, larynx, and lip. Despite significant medical advancements, HNSCC’s lethality remains a significant concern [47].
One promising therapeutic direction points to SLAM5 (CD244 or 2B4), a signaling lymphocyte activation molecule family member. This molecule is primarily found in hematopoietic cells, including Natural Killer (NK) cells, certain CD8+ αβ T cells, Dendritic Cells (DCs), and Myeloid-Derived Suppressor Cells (MDSCs). Significantly, CD244 interacts with CD48, found in most hematopoietic cells [48,49,50].
Within HNSCC patients and their mouse model counterparts, there is a marked increase in CD244 levels in tumor-infiltrating CD8+ T cells, which also correlates with heightened PD-1 expression. This elevation is seen not just in these T cells but also in DCs and MDSCs within many tumor sites. A strong correlation exists between heightened CD244 and PD-L1 levels, paired with a surge in immune-suppressive agents. Activation of CD244 in a lab setting was found to suppress the release of crucial pro-inflammatory cytokines in human DCs, and intriguingly, CD244-deficient mice demonstrated delayed HNSCC tumor progression [51].
The potential therapeutic benefits of targeting CD244 have been previously described, especially given its association with releasing immune-suppressive agents in DCs and Mo-MDSCs. This hints at a potential weakening of the immune response within the tumor environment by CD244 signaling [42]. Prior research has indicated a connection between elevated CD244 levels in CD8+ T cells and increased PD-1 expression, suggesting CD244’s role in the exhaustion of these T cells in chronic viral infections and cancer [52,53].
With respect to the myeloid tumor compartment, it is theorized that CD244 signaling augments the immune-suppressive characteristics of both DCs and MDSCs. In the case of DCs, CD244 signaling might reduce the initial activation of T cells and the stimulation of NK cells [48]. Meanwhile, in MDSCs, the presence of CD244 aligns with the suppression of specific antigen-driven CD8+ T cells [48].
5. Hepatocellular Carcinoma (HCC)
Hepatocellular Carcinoma (HCC) is the most prevalent form of primary liver cancer and poses a significant global health challenge due to its high mortality and limited therapeutic options [54]. Ranking third in cancer-related deaths worldwide, the incidence of HCC is anticipated to rise in the upcoming years [48].
SLAMF3 is uniquely underexpressed in human liver cells, suggesting its potential role in HCC development, whereas other SLAMFs have not been implicated. Notably, SLAMF3 expression is evident in 40–65% of healthy human liver cells, but this is markedly reduced in HCC cell lines [55]. Both mRNA and protein levels of SLAMF3 are considerably lower in HCC cells compared to their healthy counterparts. This observation is further supported by tumor specimens from HCC patients, where SLAMF3 was significantly less present in cancerous areas compared to the surrounding non-cancerous regions [56].
SLAMF3 has been identified as having a significant relationship with cell growth, particularly in the context of HCC. Studies have shown that when HCC cells with high SLAMF3 expression are implanted in mice, the growth of the tumor is inhibited [56]. This suggests that rather than promoting tumor growth, high SLAMF3 expression might suppress it. Additionally, liver cells with abundant SLAMF3 have decreased activity in key cellular pathways like MAPK, ERK1/2, JNK, and mTOR, which are typically associated with cell proliferation. This further underscores SLAMF3’s potential role in regulating HCC cell proliferation [55]. Based on these findings, SLAMF3 not only serves as a distinguishing biomarker between healthy and malignant liver cells but also emerges as a potential therapeutic target in HCC treatment.
6. Colorectal Cancer (CRC)
Colorectal Cancer (CRC), the most common digestive system malignancy, has both prevalence and mortality rates that eclipse many other widespread cancers [57]. Recently, there have been significant advancements in treatment for certain subtypes, particularly with the emergence of immune checkpoint inhibitors (ICIs) such as anti-PD-1 monoclonal antibodies, leading to frequent updates in treatment guidelines [58].
Also known as BLAME or CD353, SLAMF8 is a surface protein within the SLAM family. Despite its potential significance, more research is needed on the role of SLAMF8 within the tumor microenvironment [59]. Recent studies pinpoint the predominant expression of SLAMF8 in tumor-associated macrophages (TAMs), which play a pivotal role in fostering an immune-suppressive tumor milieu. These TAMs are instrumental in establishing an immune-suppressive environment around tumors [60]. With various SLAM family proteins known to influence the tumor immune landscape, their potential as immunotherapy targets becomes increasingly evident.
Further research has indicated a correlation between SLAMF8 expression and the presence of CD8-positive T cells in colorectal cancer. This association suggests that SLAMF8, like other SLAM family members, may influence the tumor’s immune milieu [61]. In essence, emerging evidence correlates SLAMF8 expression with malignancy progression, unfavorable outcomes, and the expression of distinct immune checkpoint markers in CRC. While these findings shine a light on its therapeutic potential, further investigations are imperative to confirm SLAMF8’s viability as an immunotherapy target [61].
7. Melanoma
Originating from malignant melanocytes, melanomas predominantly arise in the skin’s basal layer [62]. However, they can also manifest in unexpected regions, such as the uvea, digestive system, genitourinary tract, and the meninges—the protective layers around the brain and spinal cord [63].
A recent study employing a unique anti-hsSLAMF9 antibody explored human melanoma specimens. Findings indicated the following:
The presence of SLAMF9+ tumor-associated macrophages (TAMs) in 73.3% of human melanomas.
Notably, these macrophages were evident in 95.5% of nevi from melanoma patients and 50% from non-melanoma individuals.
SLAMF9 expression was observed in melanocytic cells of 20% of melanomas and a mere 2.3% of nevi from melanoma patients [12].
SLAMF9, a recent debutant to the immunoglobulin superfamily of receptors, is expressed on TAMs across both mouse and human melanomas. Its significant role involves modulating pro-inflammatory cytokines’ release and affecting cellular movement [12]. The widespread occurrence of SLAMF9 in both benign and malignant melanocytic formations complicates its direct linkage with malignancy. Focused research is crucial to determine SLAMF9’s reliability as a melanoma marker [12].
In summary, SLAMF9 emerges as a promising component of the immunoglobulin-receptor superfamily. Present within various melanocytic growths, its capability to regulate pro-inflammatory responses and influence macrophage movement, even devoid of internal signaling constructs, is intriguing. Delving deeper into its role, potential binding allies and initiated signaling will be pivotal. Fully grasping the role of SLAMF9+ TAMs in melanoma’s development and progression could spotlight this molecule as a crucial therapeutic target in the near future [12].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15194808
This entry is offline, you can click here to edit this entry!
