Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Severe aortic stenosis (AS) carries a poor prognosis with the onset of heart failure (HF) symptoms, and surgical or transcatheter aortic valve replacement (AVR) is its only definitive treatment. The management of AS has seen a paradigm shift with the adoption of transcatheter aortic valve replacement (TAVR), allowing for the treatment of AS in patients who would not otherwise be candidates for surgical AVR.
- aortic stenosis
- aortic valve replacement
- heart failure
1. Introduction
Valvular heart disease represents an important global health problem, and its prevalence is expected to rise due to the aging population. Aortic stenosis (AS) is the most common type of valvular heart disease in the western world, and deaths related to it have been on the rise for the last two decades [1,2]. Degenerative or calcific AS results from progressive valve thickening and calcification [3], which ultimately lead to outflow tract obstruction of the left ventricle (LV). Left untreated, severe AS leads to progressive heart failure and death. Aortic valve replacement (AVR) prolongs survival and is the only definitive therapy for severe symptomatic AS [4]. Calcific AS was associated with an estimated 151,000 global deaths in 2021 and a loss of over 2 million disability-adjusted life years [5]. The classic symptoms of AS are angina, syncope, and dyspnea. Dyspnea is an ominous sign signaling the onset of heart failure (HF) and shift from a compensated to a decompensated phase in the progression of AS.
AVR, the only definitive therapy for severe AS, improves survival and health status. Current guidelines recommend AVR for patients with severe symptomatic AS [6,7]. Among asymptomatic patients, AVR is indicated in those with severe AS with an LV ejection fraction of <50%, and in those with very high aortic valve gradients (mean gradient > 60 mmHg) and a low operative risk [6,7]. Both surgical AVR (SAVR) and transcatheter AVR (TAVR) are established therapies for AS. The decision between TAVR and SAVR depends on the patient’s age, frailty, comorbidities, and valve anatomy. While younger patients with a low operative risk may be preferentially treated with SAVR, older patients with comorbidities that increase their surgical risk, such as prior chest radiation or porcelain aorta, may be preferentially treated with TAVR [8]. Multiple randomized controlled trials have established the role of TAVR in high-, intermediate-, and low-risk surgical candidates [9,10,11]. The number of TAVRs performed yearly is on the rise in the US and exceeded the number of SAVRs performed in 2019 [12].
2. Incidence of HF Hospitalization after AVR
The outcomes of TAVR have improved over time with device improvements, refined procedural techniques, and lower-risk patient populations. A study from the Transcatheter Valve Therapy (TVT) registry demonstrated that, among 12,182 patients treated with TAVR in the United States between 2011 and 2013, the rate of HF readmission at 1 year was 14.3% and the 1-year overall mortality was 23.7% [18]. However, 1-year overall mortality has decreased substantially in recent years and is now 10% in clinical practice and <2% in recent clinical trials on low-risk patients (Figure 1) [9,10,11,12,19,20,21,22].
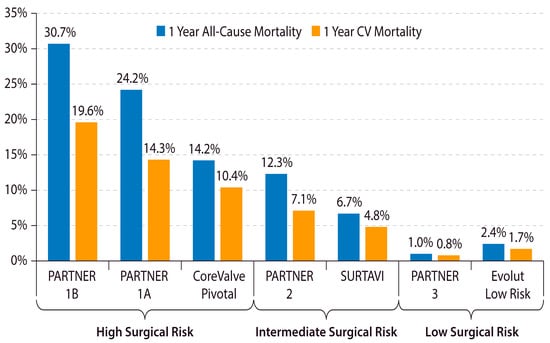
Figure 1. Declining 1-year mortality after TAVR in lower-risk Patients. The 1-year all-cause and cardiovascular mortality after TAVR in major TAVR clinical trials is shown. CV = cardiovascular. PARTNER = Placement of Aortic Transcatheter Valves. SURTAVI = Surgical Replacement and Transcatheter Aortic Valve Implantation [9,10,11,19,20,21,22].
Despite these improvements in survival post-TAVR, the incidence of HF hospitalization after TAVR remains a concern. The incidence of HF after TAVR reported in various registries and clinical trials ranges from 7 to 24% [14,16,18,23,24,25,26] (Table 1). This is comparable to the incidence of HF hospitalization in clinical trials on chronic systolic heart failure patients [27,28]. The HF rate post-AVR is higher in observational studies from registries compared to clinical trials. The difference between these trial and real-world practice HF rates may be due to the frequency of follow-ups, the intensity of medical therapy, or the Hawthorne effect, where study participants have lower rates as a result of being observed in a trial setting [29]. In the TVT Registry, HF was the most common reason for readmission within the first year after TAVR [26]. A recent post hoc analysis of 3403 TAVR and SAVR patients included in the PARTNER (Placement of Aortic Transcatheter Valves) I, II, and III trials demonstrated that HF hospitalizations within 1 year after AVR are associated with an increased mortality and worse 1-year health status, irrespective of the type of AVR (TAVR or SAVR) [14].
Table 1. Incidence of heart failure hospitalization after aortic valve replacement.
| Publication Year | Author | N | AVR Type | Design | 1-Year HF Hospitalization |
|---|---|---|---|---|---|
| 2022 | Huded et al. [14] | 3403 | SAVR and TAVR | Secondary analysis of RCTs | 6.7% |
| 2020 | Auffret et al. [30] | 808 | TAVR | Single-center retrospective | 13.6% |
| 2019 | Vemulapalli et al. [26] | 15,324 | TAVR | Multicenter retrospective | 14.2% |
| 2019 | Guedeney et al. [16] | 1139 | TAVR | Multicenter prospective | 9.2% |
| 2019 | Harbaoui et al. [24] | 409 | TAVR | Multicenter prospective | 19.9% |
| 2018 | Nazzari et al. [25] | 742 | TAVR | Multicenter prospective | 12.4% |
| 2017 | Durand et al. [23] | 546 | TAVR | Single-center retrospective | 24.1% |
| 2017 | Forcillo et al. [31] | 714 | TAVR | Single-center retrospective | 7.6% |
| 2015 | Holmes Jr et al. [18] | 12,182 | TAVR | Multicenter retrospective | 14.3% |
AVR = aortic valve replacement. HF = heart failure. RCT = randomized controlled trial. SAVR = surgical aortic valve replacement. TAVR = transcatheter aortic valve replacement.
3. The Kansas City Cardiomyopathy Questionnaire: A Tool to Evaluate HF Symptoms
The aim of AVR is to improve both longevity and health status (symptoms, physical functioning, and quality of life). Changes in health status after AVR can be accurately and reliably measured using the Kansas City Cardiomyopathy Questionnaire (KCCQ). The KCCQ was originally developed as a tool for measuring the symptoms, social and physical limitations, quality of life, and self-efficacy in HF patients and has been validated as a reliable instrument for measuring the health status of severe AS patients [32,33]. It is a 23-item self-administered questionnaire that measures 7 specific health domains relevant to HF. The domain scores and 3 summary scores are each represented on a 0–100 scale, with higher scores indicating a better health status [34]. An abbreviated 12-item version, the KCCQ-12, has been validated and has an excellent concordance with the original 23-item scale. The KCCQ overall summary score (KCCQ-OS) is commonly reported as a summary assessment of the health status of AS patients before and after AVR. To improve their interpretability, KCCQ-OS scores are often analyzed in 25-point ranges (0–24, 25–49, 50–74, and 75–100), representing very poor to poor, poor to fair, fair to good, and good to excellent health statuses [34].
Both TAVR and SAVR have shown remarkable improvements in KCCQ-OS from baseline to 1 year in recent clinical trials (Figure 2). Despite a large average treatment effect of AVR being evidenced by these clinical trial results, a substantial proportion of patients continue to remain symptomatic with HF after AVR. This discrepancy highlights the importance of identifying patients with residual HF symptoms despite a successful AVR procedure. A major advance in this area was the development of a “poor outcome” after TAVR, a concept that was introduced by Arnold et al. in 2013 [35]. In the current era, a “poor outcome” after TAVR is defined as death, KCCQ-OS of <60, or a decline in KCCQ-OS by 10 or more points from baseline to 1-year post-TAVR. This framework allows investigators to identify patients who either did not survive or are living with a low or declining health status 1 year after AVR.
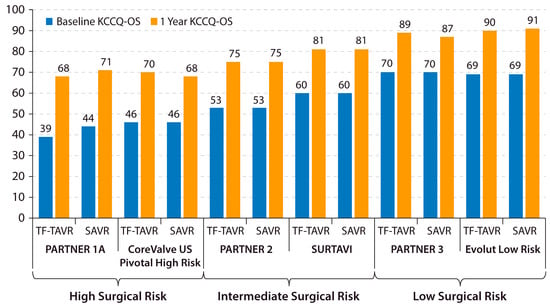
Figure 2. Changes in KCCQ-OS after AVR in major trials. Baseline and 1-year KCCQ-OS is shown for the TF-TAVR and SAVR arms of recent major AVR trials across the surgical risk spectrum. KCCQ-OS = Kansas City Cardiomyopathy Questionnaire overall summary score. PARTNER = Placement of Aortic Transcatheter Valves. SAVR = surgical aortic valve replacement. SURTAVI = Surgical Replacement and Transcatheter Aortic Valve Implantation. TF-TAVR = transfemoral transcatheter aortic valve replacement. US = United States [19,36,37,38,39].
Remarkably, in practice, a large proportion of patients meet the definition of a poor outcome 1 year after TAVR, and, although this is declining [40], the decline in poor outcomes is far less than the rate of decline in 1-year post-TAVR mortality. In 2018, the rate of poor outcomes after TAVR was 32%, with 19% of patients having a low or declining health status [12]. These data highlight that, even in the modern era of TAVR, 1 out of 3 patients die or suffer from a substantial HF symptom burden with a low or declining health status 1 year after TAVR. This observation underscores the need for strategies to better identify and treat these patients longitudinally pre- and post-AVR.
4. Risk Factors for Poor Outcome and HF after AVR
Various risk models have been developed to predict patients at risk of poor outcomes based on pre-procedure patient characteristics [41]. Among the high-risk patients treated in PARTNER I, predictors of a poor outcome at 1 year included a higher baseline creatinine level, oxygen-dependent lung disease, a lower baseline mean aortic valve gradient, a lower baseline score on the Mini-Mental Status Examination, and a shorter baseline 6 min walk test distance [41]. An updated model from the TVT Registry reported a lower baseline KCCQ-OS, a lower mean aortic valve gradient, home oxygen use, a higher baseline creatinine level, atrial fibrillation or flutter, and diabetes mellitus as significant predictors of 1-year poor outcomes [40]. A similar model was developed among the high-risk patients treated in the CoreValve US Pivotal Extreme and High-Risk trials of self-expanding TAVR devices, which identified the same key clinical variables (KCCQ-OS, mean aortic valve gradient, home oxygen, creatinine level, cognitive function, atrial fibrillation or flutter, and diabetes), but further added frailty as a component [42]. These risk models highlight that poor outcomes after AVR, either with death or a low health status, are associated with major non-cardiac comorbidities such as chronic lung disease, renal dysfunction, diabetes, and frailty.
Risk factors for HF hospitalization after TAVR have also been studied and are largely similar to the factors associated with the poor outcome metric (Table 2). A recent analysis of 3403 pooled TAVR and SAVR patients across the low-, intermediate-, and high-risk cohorts from the PARTNER trials identified a low baseline aortic valve mean gradient, atrial fibrillation or flutter, and prior coronary revascularization as factors associated with 1-year HF hospitalization [14]. Several prior registry studies have identified atrial fibrillation, renal insufficiency, a lower aortic valve mean gradient, higher surgical risk scores, diabetes mellitus, and a lower LV ejection fraction as risk factors for HF hospitalization after TAVR [16,23,24,25,43,44,45]. In addition to baseline patient characteristics, several post-procedure issues may also increase the risk of HF symptoms and hospitalization. Paravalvular regurgitation [46], patient–prosthesis mismatch [47,48], new-onset left bundle branch block, and the need for permanent pacemaker implantation [49,50] are associated with an increased incidence of HF hospitalization after TAVR.
Table 2. Established risk factors for HF hospitalization or poor outcome after AVR.
| Baseline Characteristics | Post-Procedural Characteristics |
|---|---|
| Lower baseline KCCQ-OS | Paravalvular regurgitation |
| Lower baseline aortic valve mean gradient | Patient–prosthesis mismatch |
| Higher baseline creatinine | New left bundle branch block |
| Atrial fibrillation or flutter | New permanent pacemaker |
| Diabetes mellitus | Lower 30-day KCCQ-OS |
| Oxygen-dependent lung disease | |
| Frailty | |
| Poor cognitive function |
Poor outcome defined as death, KCCQ-OS < 60, or decline of ≥ 10 points from baseline after AVR. AVR = aortic valve replacement. HF = heart failure. KCCQ = Kansas City Cardiomyopathy Questionnaire overall summary score.
There is a paucity of contemporary data evaluating the risks for HF hospitalization after SAVR. However, the aforementioned analysis of the pooled TAVR and SAVR patients treated in the PARTNER trials found that treatment type (TAVR vs. SAVR) was not associated with the risk of HF hospitalization within 1 year after AVR [14]. This finding suggests that the risk of HF hospitalization is similar between AVR types. Additionally, treatment type (TAVR vs. SAVR) was not an effect modifier on the relationship between post-AVR HF hospitalization and poor 1-year outcomes, such as death or a low health status. This finding indicates that patients did similarly poorly in terms of HF after AVR, regardless of whether their AVR type was TAVR or SAVR.
In addition to pre-procedural patient characteristics and post-procedural valve-related complications, post-procedure health status has also been shown to have important prognostic implications for patients undergoing transcatheter valve procedures. A recent study of 67,669 patients treated with either TAVR or mitral transcatheter edge-to-edge repair from the TVT registry demonstrated that both baseline and 30-day KCCQ scores were predictive of HF hospitalization and death, with the 30-day assessment having the strongest association with 1-year outcomes [51]. These results point to the utility of the 30-day post-procedure assessment to identify high-risk patients for poor outcomes and implement novel strategies for managing post-TAVR HF before poor outcomes ensue.
This entry is adapted from the peer-reviewed paper 10.3390/jcm12186048
This entry is offline, you can click here to edit this entry!
