Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The detection of animal viruses remains a formidable scientific challenge, while concurrently presenting a profoundly consequential practical concern of considerable magnitude, necessitating the development of rapid, sensitive, specific, on-site, cost-effective, and user-friendly diagnostic assays.
- electrochemical biosensor
- animal virus
- detection
1. Introduction
Viruses are a unique class of infectious, obligate intracellular parasites whose genetic material is composed of either DNA or RNA [1]. The virus particle itself is composed of a nucleocapsid, which contains the genome with the ability to replicate and a protein shell [2]. In the case of enveloped viruses, the nucleocapsid is surrounded by a lipid membrane resembling that of the host cell, which is studded with spike structures. The virus is a parasitic entity that relies on the host cell machinery to synthesize its own viral components, allowing for the successful replication and spread of the virus [3]. Viral infections pose a significant menace to both public health and animal husbandry [4][5][6]. Viruses can be transmitted through various means such as water, land, air, body fluids, and excreta, among others [7], and viruses can spread rapidly and eventually lead to the elimination or death of infected animals, resulting in significant economic losses [8][9][10].
The African Swine Fever virus (ASFV) has occurred in 22 countries worldwide since 2016, with Asia being the most affected in terms of animal losses. In China, the outbreak of ASF was first confirmed on 3 August 2018 [11]. According to the Chinese government’s website, a total of 143 cases of ASF were reported by July 2019 and more than 1.2 million pigs were culled. The estimated direct economic impact of ASF in China amounts to CNY 1 trillion, which is without considering the upstream and downstream of the industrial chain. The outbreak of ASF in Vietnam in 2019 had a severe impact on the Vietnamese pig sector, with more than 20% of the country’s pigs being culled or killed [12].
The impact of the virus is not only in the breeding of pigs but also in the poultry industry. In 2003, the Netherlands experienced an epidemic of highly pathogenic avian influenza (HPAI) caused by the H7N7 virus subtype. Thirty million birds were culled in this outbreak, about one third of the total poultry population [13]. The HPAI outbreak in Turkey from 2005 to 2006 resulted in a EUR 28 million loss for broiler producers [14]. There are other animal viruses, such as blue-ear disease in pigs [15], foot-and-mouth disease [16], Marek’s disease [17], etc. Therefore, it is important to reduce economic losses by the rapid detection of viruses.
The rising apprehension within the livestock industry regarding the emergence and dissemination of numerous animal viruses has prompted the adoption of diverse control strategies. These measures are designed to curtail the virus’s propagation and mitigate the associated losses [18]. However, effective viral detection remains a key factor in managing these pathogens. Traditional diagnostic methods such as nucleic acid amplification-based techniques [19], antigen or antibody-based assays [20], and viral isolation [21] all exhibit a number of limitations, including extended testing times, specialized equipment requirements, and technical expertise [22]. Therefore, there is an urgent need for fast, user-friendly, and field-applicable virus detection modes.
The electrochemical biosensor is an attractive platform for quick virus detection. Electrochemical sensors have proven to be an inexpensive and sensitive method, and are used to detect analytes involved in healthcare, environmental monitoring, and food packaging by diagnosing the virus before it spreads and cutting off transmission routes [23]. Traditional virus detection methods have lagged behind and are a significant cause of outbreaks. Electrochemical biosensors have heightened sensitivity and selectivity, affordability, ease of use, portability, and rapid analysis, making them suitable for real-time virus detection and overcoming the limitations of traditional detection methods [24]. Due to the scarcity of electrochemical biosensors for the rapid detection of animal viruses, the purpose of this research is to use the characteristics of electrochemical biosensors and their contributions in other fields to cite these achievements for the rapid detection of animal viruses (Figure 1).
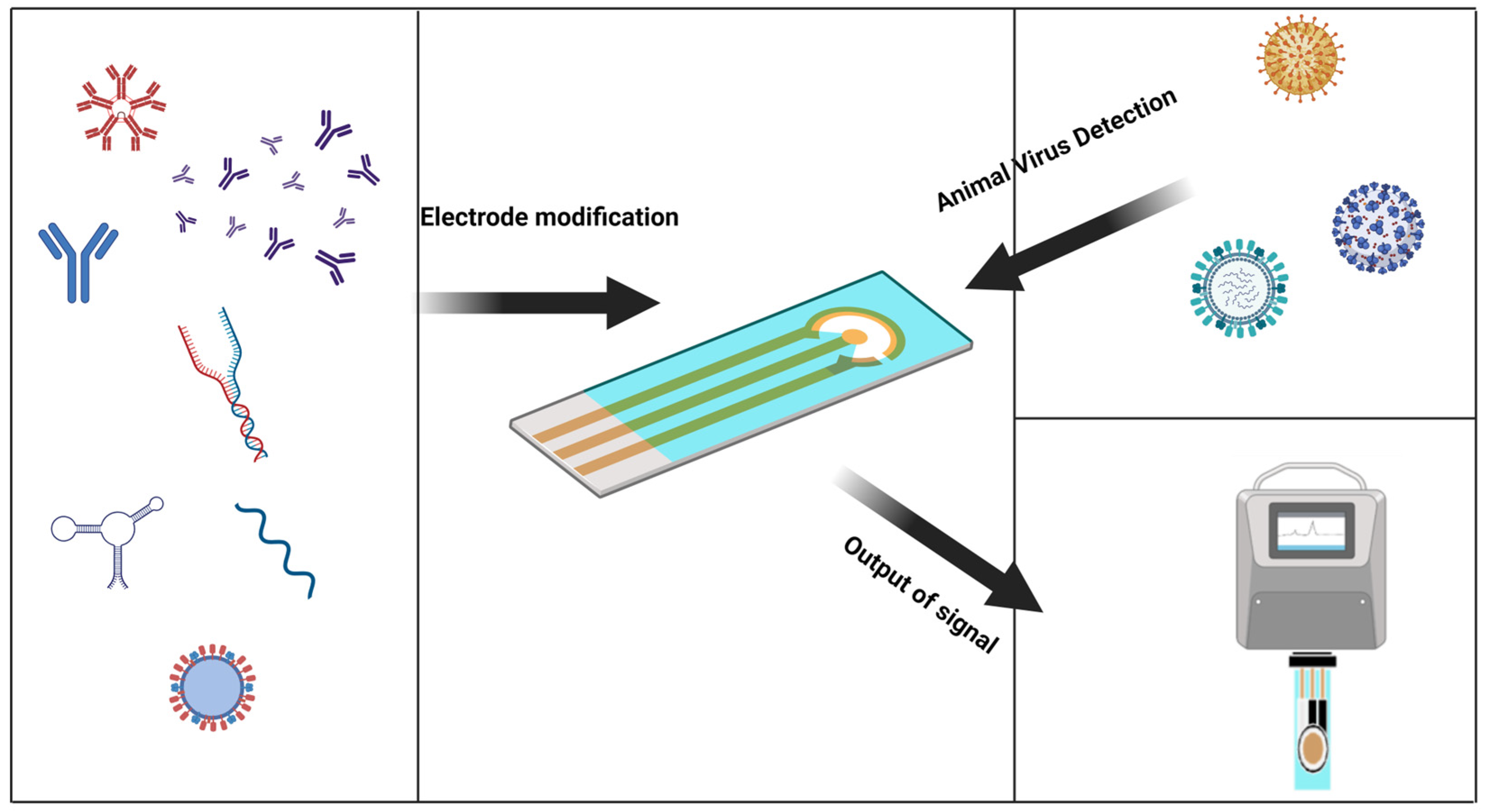
Figure 1. Graphical abstract, a general introduction to the electrochemical sensor detection process.
2. Electrochemical Biosensors
2.1. Components of Electrochemical Biosensors
Each biosensor has four parts: sample or analytes, a biorecognition element, a transducer, and a signal processing device. Biosensors are categorized based on their transducer into electrochemical, optical etc. An electrochemical biosensor is a kind of detection device that uses biological molecules including proteins, nucleic acids, etc., to specifically bind to target analytes [25] (Figure 2A). The biological material is used as the sensitive element of the electrochemical biosensor, the electrode is used as the conversion element, the potential or current is the characteristic signal, and the characteristic signal is reflected in the analytical test device on the electrochemical workstation [26]. In electrochemical experiments, a commonly employed configuration is the three-electrode system, comprising a working electrode (WE), a reference electrode (RE), and a counter electrode (CE) [27]. The WE is the place where the electrochemical reaction occurs and is the object of study, the RE is used as a reference to measure and control the system potential, and the CE is used to complete the closed circuit to achieve the electrochemical measurement. In order to better connect the biomaterial with the electrode and exert the best performance of the sensor, the help of some compounds, such as thiol compounds [28] and conductive polymers [29], is usually needed to make the electrode fully modified (Figure 2B). Biomaterials were modified onto a WE to form complexes. The electrode–biomaterial complex binds to the analyte, and usually these bindings are irreversible, such as the paired hybridization of nucleic acids and the specific binding of antigens and antibodies, combined with different detection methods of the electrochemical workstation, and the behavior of electrode modifications in the above process is recorded on the electrochemical workstation [30].
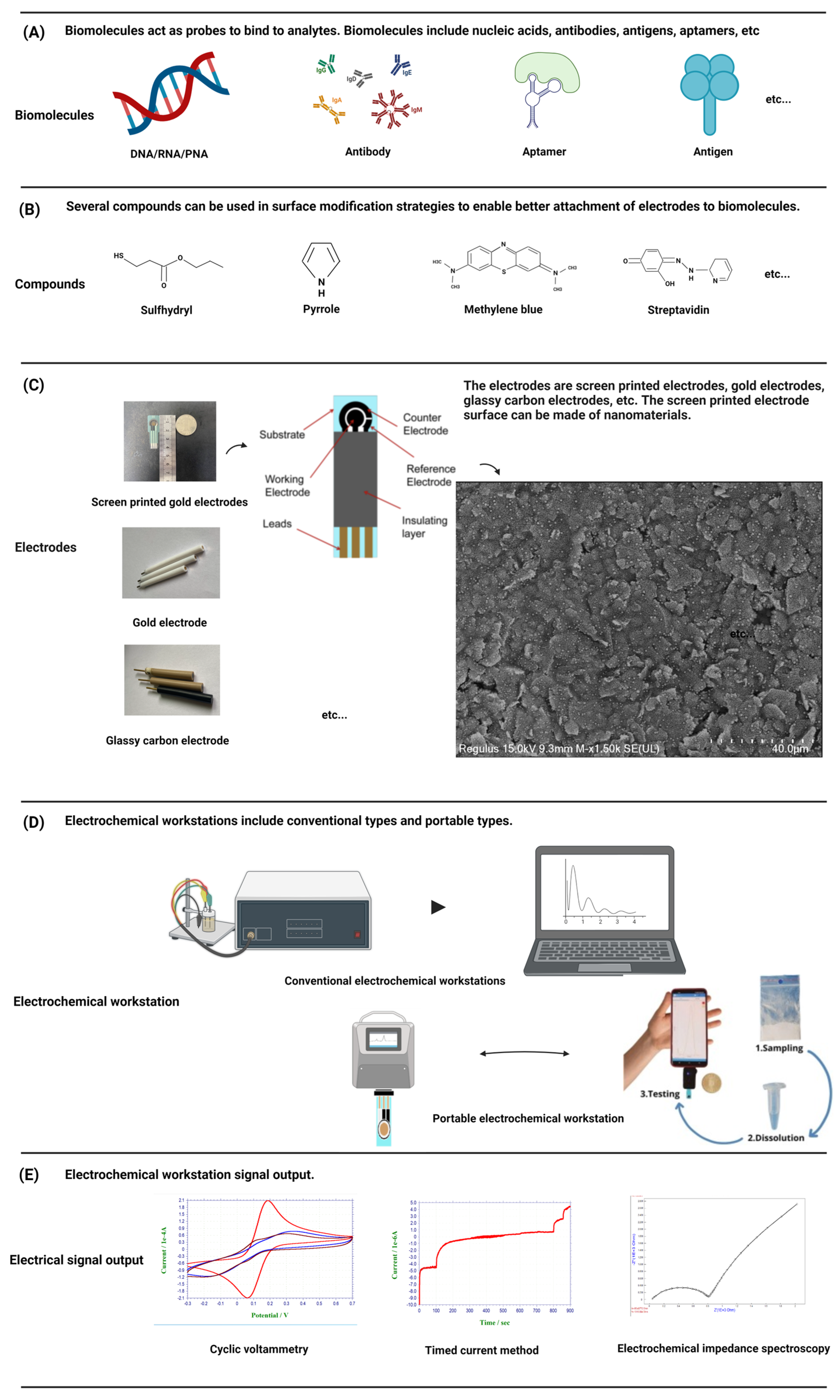
Figure 2. A schematic diagram of a standard electrochemical workstation and portable electrochemical workstation is presented. The components of the detection system, including electrodes, biomolecules, compounds, etc., and the conventional signal output are introduced.
2.1.1. Electrodes
Commonly used electrode materials are gold electrodes, glass carbon electrodes, graphene electrodes, screen-printed electrodes, and so on (Figure 2C). The gold electrode is widely favored due to its inert chemical properties and its ability to combine non-chemically with mercaptan on the surface of the gold electrode to form gold-sulfur (Au-S) bonds with self-assembled monolayers [31], which has been widely demonstrated in the field of nucleic acid hybridization. The glassy carbon electrode is one of the most widely used working electrodes. It has good conductivity, high hardness, a wide polarization range, and can be used directly as an inert electrode for anodic dissolution. Graphene, characterized by a monolayer of SP2-bonded carbon atoms arranged in a honeycomb lattice, exhibits remarkable attributes such as rapid electron transfers, remarkable thermal conductivity, and favorable biocompatibility. Its superior physical and chemical properties render graphene an ideal electronic material for advanced electrochemical sensing applications [32].
Screen-printed electrodes (SPEs) are an ideal component for sensor applications. Compared with the traditional three-electrode system, the sensor surface is modified with nanomaterials to ensure consistency and stability while greatly simplifying the experimental operation process. SPEs generally include a substrate for printed electrodes. The substrate is printed with an external insulation layer and electrode leads, and the substrate is also printed with a WE, a RE, and a CE. Each electrode is connected with the corresponding lead to form a three-electrode system [33]. Because the low-cost manufacturing technology of SPEs can be easily extended to mass production, and all types of materials can be added to screen-printing slurry, SPEs can be customized for different substrate materials, shapes, and sizes in production to meet the needs of a variety of research. Its low manufacturing cost and ease of manufacture make it possible to mass customize personalized products according to demand, making it an ideal tool in the field of quality-controlled, scientific research [34].
2.1.2. Working Stations
An electrochemical workstation is the abbreviation of the electrochemical detection system. Electrochemical workstations can be directly used to measure the steady state current on ultra-microelectrodes. After special treatment, if the workstation is connected with the micro-current amplifier and shielding box, a current of 1 pA or lower can be measured, which provides a solid foundation for electrochemical detection. Two primary categories of electrochemical workstations exist: single-channel workstations and multi-channel workstations. The difference is that multi-channel workstations can be used to detect multiple samples at the same time. At present, the electrochemical workstation is a commercial product, and different manufacturers provide different models of products with different electrochemical measurement techniques and functions. In order to meet the needs of rapid detection and be convenient to carry, a portable electrochemical workstation has been developed, which has the advantages of being small and light, easy to operate and cheap, and provides researchers with greater flexibility and convenience [35] (Figure 2D). Electrochemical biosensors can detect very small sample sizes and speed up the analysis process, making them powerful and highly sensitive devices [36]. So far, other types of sensors have also been reported, such as optical sensors [37], etc. Compared with other types of sensors, electrochemical biosensors do not require expensive equipment, save time, are convenient to carry, and are more user-friendly, especially for areas with backward economic development and scarce resources [24]. The common detection methods of electrochemical biosensors include voltammetry, amperometry, and the impedance method (Figure 2E).
2.2. Signal Analysis and Output
2.2.1. Voltammetry
Voltammetry is based on the voltage change between the electrode and the electrolyte solution [38], of which cyclic voltammetry is the most commonly used electrochemical method and is considered “spectroscopy for electrochemists”. After triangular wave scanning, the electrode completes a reduction and oxidation process. In the range of scanning potential, the electrode can undergo alternate reduction and oxidation reactions, and the resulting current–voltage curve is recorded, reflecting the steady-state response of chemical reactions triggered by electron transfers [39]. Cyclic voltammetry can be used in a faster time, has a wider potential to see the electrode where the Oxidation reduction reaction occurs, and provides a rich signal for the electrode. Cyclic voltammetry is generally used for qualitative analyses and rarely in quantitative analyses.
2.2.2. The Ampere Method
Amperometric detection relies on the alteration of currents. A fixed potential is applied to the electrochemical solution, which is enough to oxidize or reduce a certain electroactive substance in the solution. The change of the current and time is recorded to obtain the current–time curve [40][41]. The ampere method is an electrochemical analysis method to study the kinetics of electrode processes.
2.2.3. The Impedance Method
The electrochemical impedance spectra (EIS) theory and its data interpretation are very complex for researchers who are not familiar with it, such as biologists, biochemists, or materials scientists. The impedance method is an electrochemical measurement technique that employs a low-amplitude sine wave potential (or current) as a perturbation signal [42]. The reaction rate depends on the frequency, and the frequency change can distinguish the reaction rate of different substances in the solution. Due to its broad measurement frequency range, the impedance method provides access to a greater wealth of dynamic information and electrode interface structural details compared to other conventional electrochemical methods [43].
These basic electrochemical methods all play a crucial role in the signal changes of the working electrode modification process. On the basis of these methods, some more sensitive methods have evolved, such as pulse voltammetry, step voltammetry, the multi-point step chronoelectric method, etc., and a foundation has been established for more novel electrochemical biosensor models with potential applications.
3. Advantages and Limitations of Electrochemical Biosensors for Virus Detection
3.1. Advantages
The advantages of an electrochemical biosensor in virus detection are its short detection time and convenience, the fact that it is cheap and simple, and its real-time detection [44]. Usually, the virus is diagnosed in the laboratory by virus isolation, PCR, ELISA, and other methods. The accuracy and practicability of these traditional methods are beyond doubt, which have left a strong mark in the history of the human fight against viruses. However, these methods require specialized personnel, expensive equipment, and testing lags [45]. Due to these limitations, the detection of viruses by electrochemical biosensors has come to its final stage in history. Table 1 shows the comparison of virus detection by conventional methods and electrochemical biosensors.
Table 1. Comparison of virus detection methods.
| Methods | Time Required | Convenience | On-Site Detection | Linear Range | LOD | References |
|---|---|---|---|---|---|---|
| Virus isolation | 2–3 Days | Cannot be carried | No | / | / | |
| PCR | 1–2 h | Cannot be carried | No | / | 2.52 × 101 copies/µL | [46] |
| / | 10 CFU/ML | [47] | ||||
| ELISA | 6–8 h | Cannot be carried | No | / | 3.675 × 104 copies/µL | [48] |
| 0.5 × 10−15–5.0 × 10 −6 g/ML | 0.5 × 10−15 g/ML | [49] | ||||
| Electrochemical biosensor | 10–36 min | Is portable | Yes | 1.176 to 4.825 μg/mL | 3.569 × 101 ng/mL | [50] |
| 1–1 × 103 pfu/mL | 1 pfu/mL | [51] |
On the other hand, the popularity of electrochemical biosensors also benefits from the maturity of SPEs. SPEs have long been considered the most promising analytical tool in electrochemical detection. Compared with traditional electrodes, an SPE avoids the polishing, cleaning, and activation required for other solid electrodes and greatly simplifies the experimental process. Commercial SPEs have a high versatility, which is also one of the advantages of SPEs [52][53]. Because the low-cost manufacturing technology of SPEs can be easily extended to mass production and all types of materials can be added to screen-printing slurry, SPEs can be customized for different substrate materials, shapes, and sizes in production to meet the needs of a variety of research. At the same time, SPEs are small, powerful, low-cost, and maintenance-free and have been widely used in electrochemical research in environmental monitoring [54][55][56], clinical diagnoses [57][58], drug analyses [59], and food detection [53][60][61], which has promoted the development of electrochemical biosensors. The wide application of nanomaterials is also the cause of the rapid development of electrochemical sensors. We all know the characteristics of nanomaterials, including their small size effect, surface and interface effect, quantum size effect, etc. Gold and carbon nanomaterials are commonly used in the field of electrochemistry. These nanomaterials can be used as nanostructured electrodes for the amplification of electrical signals, thereby improving detection sensitivity [62][63]. They can also be used to prepare signal labels such as AuNPs [64] and can also be used as a platform for highly conductive nanostructures such as graphene, graphene oxide, carbon nanotubes, gold nanometers, etc., to obtain an extremely low detection limit [65].
3.2. Limitations
The advantages of electrochemical biosensors, such as their being fast, convenient, and cheap, have indeed made them more and more prominent in the diagnostic industry, although some electrochemical biosensors are still in the development and testing stages. The current problems faced by electrochemical biosensors are mainly the following aspects. The first is accuracy and reproducibility, and analyte concentration becomes increasingly important when moving target analyte testing from a clean laboratory with a controlled environment to the field. Here, many external environmental factors can contribute to significant differences in signal strength, such as the temperature, humidity, the sample volume, the electrode surface area, non-calibrated instruments, and contamination. In response to this problem, corresponding coping strategies have been reported to improve the accuracy and reproducibility of biosensors through ratio electrochemistry [66]. Secondly, the limited shelf life and stability of the biometric components as well as non-specific binding are still the biggest biosensor limitations, and corresponding strategies have been reported to overcome and reduce these aspects. Finally, the concentration used in the sensor component design is trace and requires precise operations; therefore, it is only through a reasonable design and rigorous testing that biosensors can be transferred from the laboratory to the field.
This entry is adapted from the peer-reviewed paper 10.3390/ani13193141
References
- Pellett, P.E.; Mitra, S.; Holland, T.C. Basics of virology. Handb. Clin. Neurol. 2014, 123, 45–66.
- San, M.C. Virus maturation. Adv. Exp. Med. Biol. 2019, 1215, 129–158.
- Kalia, M.; Jameel, S. Virus entry paradigms. Amino Acids 2011, 41, 1147–1157.
- Liu, Q.; Wang, H.Y. Porcine enteric coronaviruses: An updated overview of the pathogenesis, prevalence, and diagnosis. Vet. Res. Commun. 2021, 45, 75–86.
- Abd, E.M.; El-Saadony, M.T.; Alqhtani, A.H.; Swelum, A.A.; Salem, H.M.; Elbestawy, A.R.; Noreldin, A.E.; Babalghith, A.O.; Khafaga, A.F.; Hassan, M.I.; et al. The relationship among avian influenza, gut microbiota and chicken immunity: An updated overview. Poult. Sci. 2022, 101, 102021.
- Labadie, T.; Batejat, C.; Leclercq, I.; Manuguerra, J.C. Historical discoveries on viruses in the environment and their impact on public health. Intervirology 2020, 63, 17–32.
- Vidic, J.; Manzano, M.; Chang, C.; Jaffrezic-Renault, N. Advanced biosensors for detection of pathogens related to livestock and poultry. Vet. Res. 2017, 48, 11.
- Neumann, E.J.; Kliebenstein, J.B.; Johnson, C.D.; Mabry, J.W.; Bush, E.J.; Seitzinger, A.H.; Green, A.L.; Zimmerman, J.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 2005, 227, 385–392.
- Liu, S.; Zhuang, Q.; Wang, S.; Jiang, W.; Jin, J.; Peng, C.; Hou, G.; Li, J.; Yu, J.; Yu, X.; et al. Control of avian influenza in China: Strategies and lessons. Transbound. Emerg. Dis. 2020, 67, 1463–1471.
- Nakada, S.; Fujimoto, Y.; Kohara, J.; Makita, K. Economic losses associated with mastitis due to bovine leukemia virus infection. J. Dairy. Sci. 2023, 106, 576–588.
- Liu, Y.; Zhang, X.; Qi, W.; Yang, Y.; Liu, Z.; An, T.; Wu, X.; Chen, J. Prevention and control strategies of African swine fever and progress on pig farm repopulation in China. Viruses 2021, 13, 2552.
- Nguyen-Thi, T.; Pham-Thi-Ngoc, L.; Nguyen-Ngoc, Q.; Dang-Xuan, S.; Lee, H.S.; Nguyen-Viet, H.; Padungtod, P.; Nguyen-Thu, T.; Nguyen-Thi, T.; Tran-Cong, T.; et al. An assessment of the economic impacts of the 2019 African swine fever outbreaks in Vietnam. Front. Vet. Sci. 2021, 8, 686038.
- Stegeman, A.; Bouma, A.; Elbers, A.R.W.; de Jong, M.C.M.; Nodelijk, G.; de Klerk, F.; Koch, G.; van Boven, M. Avian influenza a virus (h7n7) epidemic in the Netherlands in 2003: Course of the epidemic and effectiveness of control measures. J. Infect. Dis. 2004, 190, 2088–2095.
- Aral, Y.; Yalcin, C.; Cevger, Y.; Sipahi, C.; Sariozkan, S. Financial effects of the highly pathogenic avian influenza outbreaks on the Turkish broiler producers. Poult. Sci. 2010, 89, 1085–1088.
- Mcorist, S.; Khampee, K.; Guo, A. Modern pig farming in the people’s republic of China: Growth and veterinary challenges. Rev. Sci. Tech. Off. Int. Epizoot. 2011, 30, 961–968.
- Poonsuk, K.; Gimenez-Lirola, L.; Zimmerman, J.J. A review of foot-and-mouth disease virus (FMDV) testing in livestock with an emphasis on the use of alternative diagnostic specimens. Anim. Health Res. Rev. 2018, 19, 100–112.
- Kennedy, D.A.; Cairns, C.; Jones, M.J.; Bell, A.S.; Salathe, R.M.; Baigent, S.J.; Nair, V.K.; Dunn, P.A.; Read, A.F. Industry-wide surveillance of Marek’s disease virus on commercial poultry farms. Avian Dis. 2017, 61, 153–164.
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. B-Biol. Sci. 2010, 365, 2853–2867.
- Monis, P.T.; Giglio, S. Nucleic acid amplification-based techniques for pathogen detection and identification. Infect. Genet. Evol. 2006, 6, 2–12.
- Day, M.J. Introduction to antigen and antibody assays. Top. Companion Anim. Med. 2015, 30, 128–131.
- Leland, D.S.; Ginocchio, C.C. Role of cell culture for virus detection in the age of technology. Clin. Microbiol. Rev. 2007, 20, 49–78.
- Babaei, A.; Rafiee, N.; Taheri, B.; Sohrabi, H.; Mokhtarzadeh, A. Recent advances in early diagnosis of viruses associated with gastroenteritis by biosensors. Biosensors 2022, 12, 499.
- Aydin, E.B.; Aydin, M.; Sezgintürk, M.K. Advances in electrochemical immunosensors. Advan. Clin. Chem. 2019, 92, 1–57.
- Manring, N.; Ahmed, M.M.N.; Tenhoff, N.; Smeltz, J.L.; Pathirathna, P. Recent advances in electrochemical tools for virus detection. Anal. Chem. 2022, 94, 7149–7157.
- Xiang, Y.; Lu, Y. Portable and quantitative detection of protein biomarkers and small molecular toxins using antibodies and ubiquitous personal glucose meters. Anal. Chem. 2012, 84, 4174–4178.
- Banakar, M.; Hamidi, M.; Khurshid, Z.; Zafar, M.S.; Sapkota, J.; Azizian, R.; Rokaya, D. Electrochemical biosensors for pathogen detection: An updated review. Biosensors 2022, 12, 927.
- Eom, H.; Kang, J.; Jang, S.; Kwon, O.; Choi, S.; Shin, J.; Nam, I. Evaluating the electrochemical properties of supercapacitors using the three-electrode system. J. Vis. Exp. 2022, 179, e63319.
- Lereau, M.; Fournier-Wirth, C.; Mayen, J.; Farre, C.; Meyer, A.; Dugas, V.; Cantaloube, J.F.; Chaix, C.; Vasseur, J.J.; Morvan, F. Development of innovative and versatile polythiol probes for use on ELOSA or electrochemical biosensors: Application in hepatitis c virus genotyping. Anal. Chem. 2013, 85, 9204–9212.
- El-Said, W.A.; Abdelshakour, M.; Choi, J.H.; Choi, J.W. Application of conducting polymer nanostructures to electrochemical biosensors. Molecules 2020, 25, 307.
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763.
- Inkpen, M.S.; Liu, Z.F.; Li, H.; Campos, L.M.; Neaton, J.B.; Venkataraman, L. Non-chemisorbed gold–sulfur binding prevails in self-assembled monolayers. Nat. Chem. 2019, 11, 351–358.
- Lawal, A.T. Synthesis and utilisation of graphene for fabrication of electrochemical sensors. Talanta 2015, 131, 424–443.
- Costa-Rama, E.; Fernandez-Abedul, M.T. Paper-based screen-printed electrodes: A new generation of low-cost electroanalytical platforms. Biosensors 2021, 11, 51.
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-printed electrodes modified with metal nanoparticles for small molecule sensing. Biosensors 2020, 10, 9.
- Ji, D.; Liu, L.; Li, S.; Chen, C.; Lu, Y.; Wu, J.; Liu, Q. Smartphone-based cyclic voltammetry system with graphene modified screen printed electrodes for glucose detection. Biosens. Bioelectron. 2017, 98, 449–456.
- Mohamad Nor, N.; Ridhuan, N.S.; Abdul Razak, K. Progress of enzymatic and non-enzymatic electrochemical glucose biosensor based on nanomaterial-modified electrode. Biosensors 2022, 12, 1136.
- Gruber, P.; Marques, M.; Szita, N.; Mayr, T. Integration and application of optical chemical sensors in microbioreactors. Lab. Chip 2017, 17, 2693–2712.
- Venton, B.J.; Cao, Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 2020, 145, 1158–1168.
- Agrawal, K.; Naik, A.A.; Chaudhary, S.; Parvatalu, D.; Santhanam, V. Prudent practices in ex situ durability analysis using cyclic voltammetry for platinum-based electrocatalysts. Chem. Asian J. 2021, 16, 3311–3325.
- Yashin, Y.I.; Nemzer, B.V.; Ryzhnev, V.Y.; Yashin, A.Y.; Chernousova, N.I.; Fedina, P.A. Creation of a databank for content of antioxidants in food products by an amperometric method. Molecules 2010, 15, 7450–7466.
- Kumar, H.; Rani, R. Development of biosensors for the detection of biological warfare agents: Its issues and challenges. Sci. Prog. 2013, 96, 294–308.
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578.
- Khan, M.; Hasan, M.R.; Hossain, S.I.; Ahommed, M.S.; Daizy, M. Ultrasensitive detection of pathogenic viruses with electrochemical biosensor: State of the art. Biosens. Bioelectron. 2020, 166, 112431.
- Gattani, A.; Singh, S.V.; Agrawal, A.; Khan, M.H.; Singh, P. Recent progress in electrochemical biosensors as point of care diagnostics in livestock health. Anal. Biochem. 2019, 579, 25–34.
- Brazaca, L.C.; Dos, S.P.; de Oliveira, P.R.; Rocha, D.P.; Stefano, J.S.; Kalinke, C.; Abarza, M.R.; Bonacin, J.A.; Janegitz, B.C.; Carrilho, E. Biosensing strategies for the electrochemical detection of viruses and viral diseases—A review. Anal. Chim. Acta 2021, 1159, 338384.
- Liu, H.; Shi, K.; Zhao, J.; Yin, Y.; Chen, Y.; Si, H.; Qu, S.; Long, F.; Lu, W. Development of a one-step multiplex qRT-PCR assay for the detection of African swine fever virus, classical swine fever virus and atypical porcine pestivirus. BMC Vet. Res. 2022, 18, 43.
- Roig, A.P.; Carmona-Salido, H.; Sanjuan, E.; Fouz, B.; Amaro, C. A multiplex PCR for the detection of vibrio vulnificus hazardous to human and/or animal health from seafood. Int. J. Food Microbiol. 2022, 377, 109778.
- Li, M.; Sun, L.; Ma, Y.; Fei, D.; Ma, M. Development of a sandwich ELISA for the detection of Chinese sacbrood virus infection. Arch. Virol. 2020, 165, 1551–1556.
- Oh, S.; Kim, J.; Tran, V.T.; Lee, D.K.; Ahmed, S.R.; Hong, J.C.; Lee, J.; Park, E.Y.; Lee, J. Magnetic nanozyme-linked immunosorbent assay for ultrasensitive influenza a virus detection. ACS Appl. Mater. Interfaces 2018, 10, 12534–12543.
- De Castro, A.; Kochi, L.T.; Flauzino, J.; Soares, M.; Alves, V.A.; Da, S.L.; Madurro, J.M.; Brito-Madurro, A.G. Electrochemical biosensor for sensitive detection of hepatitis b in human plasma. Appl. Biochem. Biotechnol. 2022, 194, 2604–2619.
- Cheng, M.S.; Ho, J.S.; Tan, C.H.; Wong, J.P.; Ng, L.C.; Toh, C.S. Development of an electrochemical membrane-based nanobiosensor for ultrasensitive detection of dengue virus. Anal. Chim. Acta 2012, 725, 74–80.
- Couto, R.A.S.; Lima, J.L.F.C.; Quinaz, M.B. Recent developments, characteristics and potential applications of screen-printed electrodes in pharmaceutical and biological analysis. Talanta 2016, 146, 801–814.
- Torre, R.; Costa-Rama, E.; Nouws, H.P.A.; Delerue-Matos, C. Screen-printed electrode-based sensors for food spoilage control: Bacteria and biogenic amines detection. Biosensors 2020, 10, 139.
- Pérez-Fernández, B.; Costa-García, A.; Muñiz, A.D.L.E. Electrochemical (bio)sensors for pesticides detection using screen-printed electrodes. Biosensors 2020, 10, 32.
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719.
- Rishpon, J. Electrochemical biosensors for environmental monitoring. Rev. Environ. Health 2002, 17, 219–247.
- Yunus, M.H.; Yusof, N.A.; Abdullah, J.; Sulaiman, Y.; Ahmad Raston, N.H.; Md Noor, S.S. Simultaneous amperometric aptasensor based on diazonium grafted screen-printed carbon electrode for detection of cfp10 and mpt64 biomarkers for early tuberculosis diagnosis. Biosensors 2022, 12, 996.
- Pothipor, C.; Aroonyadet, N.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. A highly sensitive electrochemical microrna-21 biosensor based on intercalating methylene blue signal amplification and a highly dispersed gold nanoparticles/graphene/polypyrrole composite. Analyst 2021, 146, 2679–2688.
- Wang, C.; Xu, Y.; Zhao, X.; Li, S.; Qian, Q.; Wang, W.; Mi, X. A double-tetrahedral DNA framework based electrochemical biosensor for ultrasensitive detection and release of circulating tumor cells. Analyst 2021, 146, 6474–6481.
- Sullivan, C.; Lu, D.; Senecal, A.; Kurup, P. Voltammetric detection of arsenic (iii) using gold nanoparticles modified carbon screen printed electrodes: Application for facile and rapid analysis in commercial apple juice. Food Chem. 2021, 352, 129327.
- Abd-Rabboh, H.; Amr, A.; Naglah, A.M.; Almehizia, A.A.; Kamel, A.H. Effective screen-printed potentiometric devices modified with carbon nanotubes for the detection of chlorogenic acid: Application to food quality monitoring. RSC Adv. 2021, 11, 38774–38781.
- Ding, L.; Bond, A.M.; Zhai, J.; Zhang, J. Utilization of nanoparticle labels for signal amplification in ultrasensitive electrochemical affinity biosensors: A review. Anal. Chim. Acta 2013, 797, 1–12.
- Wang, X.; Lu, D.; Liu, Y.; Wang, W.; Ren, R.; Li, M.; Liu, D.; Liu, Y.; Liu, Y.; Pang, G. Electrochemical signal amplification strategies and their use in olfactory and taste evaluation. Biosensors 2022, 12, 566.
- Lopez-Marzo, A.M.; Hoyos-De-La-Torre, R.; Baldrich, E. Nano(3)/NaCl oxidant and polyethylene glycol (peg) capped gold nanoparticles (AuNPs) as a novel green route for AuNPs detection in electrochemical biosensors. Anal. Chem. 2018, 90, 4010–4018.
- Hashemzadeh, H.; Khadivi-Khanghah, Z.; Allahverdi, A.; Hadipour, M.M.; Saievar-Iranizad, E.; Naderi-Manesh, H. A novel label-free graphene oxide nano-wall surface decorated with gold nano-flower biosensor for electrochemical detection of brucellosis antibodies in human serum. Talanta Open 2023, 7, 100215.
- Spring, S.A.; Goggins, S.; Frost, C.G. Ratiometric electrochemistry: Improving the robustness, reproducibility and reliability of biosensors. Molecules 2021, 26, 2130.
This entry is offline, you can click here to edit this entry!
