Neuroinflammation is a complex biological process that typically originates as a protective response in the brain. This inflammatory process is triggered by the release of pro-inflammatory substances like cytokines, prostaglandins, and reactive oxygen and nitrogen species from stimulated endothelial and glial cells, including those with pro-inflammatory functions, in the outer regions. While neuronal inflammation is common in various central nervous system disorders, the specific inflammatory pathways linked with different immune-mediated cell types and the various factors influencing the blood-brain barrier significantly contribute to disease-specific characteristics.
- neuroinflammation
- proinflammatory cytokines
- endocannabinoid system
- microglia
1. Introduction
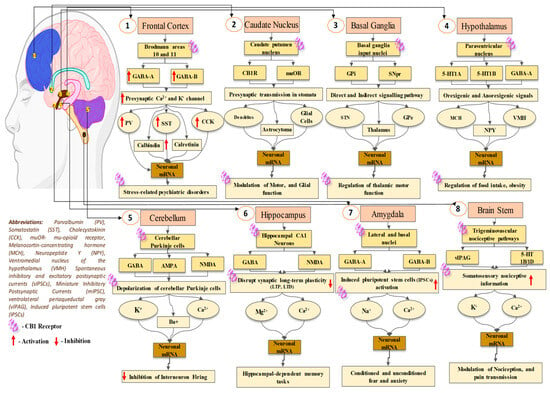
2. Establishment of the Cannabinoid Receptor 1 (CB1) in Neuronal Tissues
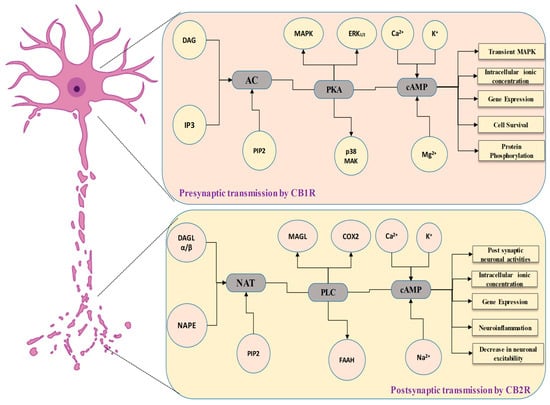
3. Cannabinoid Receptor 1 (CB1) in the Communication of Neuronal Signaling Pathways
4. Role of Cannabinoid Receptor 1 (CB1) in the Modulation of Neuronal Physiology
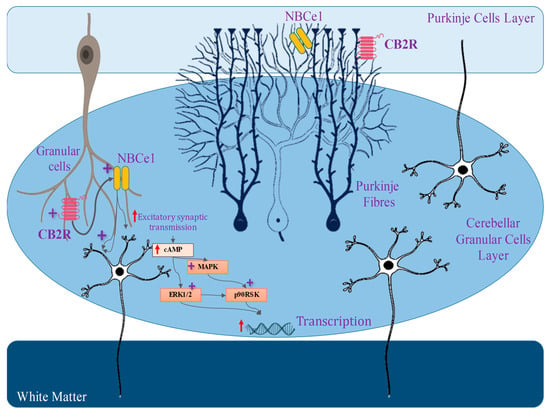
5. The Involvement of the Cannabinoid Receptor 1 (CB1) in Synaptic Modulation
6. Cannabinoid Receptor 1 (CB1) Gene Expression in the Brain
7. Establishment of the Cannabinoid Receptor 2 (CB2) in Neuronal Tissues
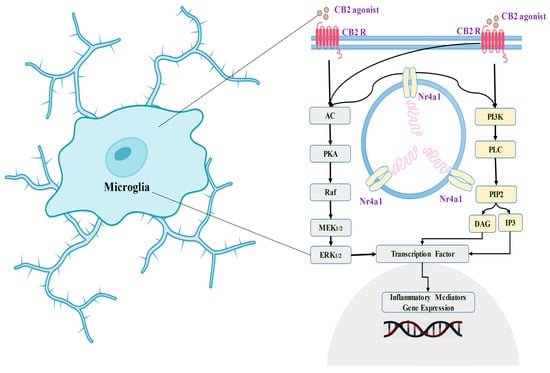
8. Cannabinoid Receptor 2 (CB2) in the Communication of Neuronal Signaling Pathways
9. Role of Cannabinoid Receptor 2 (CB2) in the Modulation of Neuronal Physiology
10. The Involvement of the Cannabinoid Receptor 2 (CB2) in Synaptic Modulation
11. Cannabinoid Receptor 2 (CB2) Gene Expression in the Brain
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11102642
References
- Kelly, R.; Joers, V.; Tansey, M.G.; McKernan, D.P.; Dowd, E. Microglial Phenotypes and Their Relationship to the Cannabinoid System: Therapeutic Implications for Parkinson’s Disease. Molecules 2020, 25, 453.
- Morena, M.; Leitl, K.D.; Vecchiarelli, H.A.; Gray, J.M.; Campolongo, P.; Hill, M.N. Emotional Arousal State Influences the Ability of Amygdalar Endocannabinoid Signaling to Modulate Anxiety. Neuropharmacology 2016, 111, 59–69.
- Vecchiarelli, H.A.; Morena, M.; Keenan, C.M.; Chiang, V.; Tan, K.; Qiao, M.; Leitl, K.; Santori, A.; Pittman, Q.J.; Sharkey, K.A. Comorbid Anxiety-like Behavior in a Rat Model of Colitis Is Mediated by an Upregulation of Corticolimbic Fatty Acid Amide Hydrolase. Neuropsychopharmacology 2021, 46, 992–1003.
- Šimončičová, E.; de Andrade, E.G.; Vecchiarelli, H.A.; Awogbindin, I.O.; Delage, C.I.; Tremblay, M.-È. Present and Future of Microglial Pharmacology. Trends Pharmacol. Sci. 2022, 43, 669–685.
- St-Pierre, M.-K.; VanderZwaag, J.; Loewen, S.; Tremblay, M.-È. All Roads Lead to Heterogeneity: The Complex Involvement of Astrocytes and Microglia in the Pathogenesis of Alzheimer’s Disease. Front. Cell. Neurosci. 2022, 16, 932572.
- Pertwee, R.G. Pharmacology of Cannabinoid CB1 and CB2 Receptors. Pharmacol. Ther. 1997, 74, 129–180.
- Shire, D.; Carillon, C.; Kaghad, M.; Calandra, B.; Rinaldi-Carmona, M.; Le Fur, G.; Caput, D.; Ferrara, P. An Amino-Terminal Variant of the Central Cannabinoid Receptor Resulting from Alternative Splicing. J. Biol. Chem. 1996, 271, 33706.
- Rinaldi-Carmona, M.; Calandra, B.; Shire, D.; Bouaboula, M.; Oustric, D.; Barth, F.; Casellas, P.; Ferrara, P.; Le Fur, G. Characterization of Two Cloned Human CB1 Cannabinoid Receptor Isoforms. J. Pharmacol. Exp. Ther. 1996, 278, 871–878.
- Griffin, G.; Wray, E.J.; Tao, Q.; McAllister, S.D.; Rorrer, W.K.; Aung, M.; Martin, B.R.; Abood, M.E. Evaluation of the Cannabinoid CB2 Receptor-Selective Antagonist, SR144528: Further Evidence for Cannabinoid CB2 Receptor Absence in the Rat Central Nervous System. Eur. J. Pharmacol. 1999, 377, 117–125.
- Skaper, S.D.; Buriani, A.; Dal Toso, R.; Petrelli, L.; Romanello, S.; Facci, L.; Leon, A. The ALIAmide Palmitoylethanolamide and Cannabinoids, but Not Anandamide, Are Protective in a Delayed Postglutamate Paradigm of Excitotoxic Death in Cerebellar Granule Neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 3984–3989.
- Howlett, A.C. Pharmacology of Cannabinoid Receptors. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 607–634.
- Greenamyre, J.T.; Young, A.B.; Penney, J.B. Quantitative Autoradiographic Distribution of L- Glutamate-Binding Sites in Rat Central Nervous System. J. Neurosci. 1984, 4, 2133–2144.
- Bowery, N.G.; Hudson, A.L.; Price, G.W. GABAA and GABAB Receptor Site Distribution in the Rat Central Nervous System. Neuroscience 1987, 20, 365–383.
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA 1990, 87, 1932–1936.
- Pettit, D.A.D.; Harrison, M.P.; Olson, J.M.; Spencer, R.F.; Cabral, G.A. Immunohistochemical Localization of the Neural Cannabinoid Receptor in Rat Brain. J. Neurosci. Res. 1998, 51, 391–402.
- Tsou, K.; Brown, S.; Sañudo-Peña, M.C.; Mackie, K.; Walker, J.M. Immunohistochemical Distribution of Cannabinoid CB1 Receptors in the Rat Central Nervous System. Neuroscience 1998, 83, 393–411.
- Moldrich, G.; Wenger, T. Localization of the CB1 Cannabinoid Receptor in the Rat Brain. An Immunohistochemical Study☆. Peptides 2000, 21, 1735–1742.
- Egertová, M.; Elphick, M.R. Localisation of Cannabinoid Receptors in the Rat Brain Using Antibodies to the Intracellular C-terminal Tail of CB1. J. Comp. Neurol. 2000, 422, 159–171.
- Tsou, K.; Mackie, K.; Sanudo-Pena, M.C.; Walker, J.M. Cannabinoid CB1 Receptors Are Localized Primarily on Cholecystokinin-Containing GABAergic Interneurons in the Rat Hippocampal Formation. Neuroscience 1999, 93, 969–975.
- Hájos, N.; Katona, I.; Naiem, S.S.; Mackie, K.; Ledent, C.; Mody, I.; Freund, T.F. Cannabinoids Inhibit Hippocampal GABAergic Transmission and Network Oscillations. Eur. J. Neurosci. 2000, 12, 3239–3249.
- Katona, I.; Sperlagh, B.; Maglóczky, Z.; Santha, E.; Köfalvi, A.; Czirjak, S.; Mackie, K.; Vizi, E.S.; Freund, T.F. GABAergic Interneurons Are the Targets of Cannabinoid Actions in the Human Hippocampus. Neuroscience 2000, 100, 797–804.
- Katona, I.; Rancz, E.A.; Acsády, L.; Ledent, C.; Mackie, K.; Hájos, N.; Freund, T.F. Distribution of CB1 Cannabinoid Receptors in the Amygdala and Their Role in the Control of GABAergic Transmission. J. Neurosci. 2001, 21, 9506–9518.
- Marsicano, G.; Lutz, B. Expression of the Cannabinoid Receptor CB1 in Distinct Neuronal Subpopulations in the Adult Mouse Forebrain. Eur. J. Neurosci. 1999, 11, 4213–4225.
- Irving, A.J.; Coutts, A.A.; Harvey, J.; Rae, M.G.; Mackie, K.; Bewick, G.S.; Pertwee, R.G. Functional Expression of Cell Surface Cannabinoid CB1 Receptors on Presynaptic Inhibitory Terminals in Cultured Rat Hippocampal Neurons. Neuroscience 2000, 98, 253–262.
- Childers, S.R.; Deadwyler, S.A. Role of Cyclic AMP in the Actions of Cannabinoid Receptors. Biochem. Pharmacol. 1996, 52, 819–827.
- Twitchell, W.; Brown, S.; Mackie, K. Cannabinoids Inhibit N- and P/Q-Type Calcium Channels in Cultured Rat Hippocampal Neurons. J. Neurophysiol. 1997, 78, 43–50.
- Mu, J.; Zhuang, S.; Kirby, M.T.; Hampson, R.E.; Deadwyler, S.A. Cannabinoid Receptors Differentially Modulate Potassium A and D Currents in Hippocampal Neurons in Culture. J. Pharmacol. Exp. Ther. 1999, 291, 893–902.
- Schweitzer, P. Cannabinoids Decrease the K+ M-Current in Hippocampal CA1 Neurons. J. Neurosci. 2000, 20, 51–58.
- Glass, M.; Felder, C.C. Concurrent Stimulation of Cannabinoid CB1 and Dopamine D2 Receptors Augments CAMP Accumulation in Striatal Neurons: Evidence for a Gs Linkage to the CB1 Receptor. J. Neurosci. 1997, 17, 5327–5333.
- Bouaboula, M.; Poinot-Chazel, C.; Bourrie, B.; Canat, X.; Calandra, B.; Rinaldi-Carmona, M.; Le Fur, G.; Casellas, P. Activation of Mitogen-Activated Protein Kinases by Stimulation of the Central Cannabinoid Receptor CB1. Biochem. J. 1995, 312, 637–641.
- Rueda, D.; Galve-Roperh, I.; Haro, A.; Guzmán, M. The CB1 Cannabinoid Receptor Is Coupled to the Activation of C-Jun N-Terminal Kinase. Mol. Pharmacol. 2000, 58, 814–820.
- Netzeband, J.G.; Conroy, S.M.; Parsons, K.L.; Gruol, D.L. Cannabinoids Enhance NMDA-Elicited Ca2+ Signals in Cerebellar Granule Neurons in Culture. J. Neurosci. 1999, 19, 8765–8777.
- Rinaldi-Carmona, M.; Le Duigou, A.; Oustric, D.; Barth, F.; Bouaboula, M.; Carayon, P.; Casellas, P.; Le Fur, G. Modulation of CB1 Cannabinoid Receptor Functions after a Long-Term Exposure to Agonist or Inverse Agonist in the Chinese Hamster Ovary Cell Expression System. J. Pharmacol. Exp. Ther. 1998, 287, 1038–1047.
- Coutts, A.A.; Anavi-Goffer, S.; Ross, R.A.; MacEwan, D.J.; Mackie, K.; Pertwee, R.G.; Irving, A.J. Agonist-Induced Internalization and Trafficking of Cannabinoid CB1 Receptors in Hippocampal Neurons. J. Neurosci. 2001, 21, 2425–2433.
- Jin, W.; Brown, S.; Roche, J.P.; Hsieh, C.; Celver, J.P.; Kovoor, A.; Chavkin, C.; Mackie, K. Distinct Domains of the CB1 Cannabinoid Receptor Mediate Desensitization and Internalization. J. Neurosci. 1999, 19, 3773–3780.
- Hsieh, C.; Brown, S.; Derleth, C.; Mackie, K. Internalization and Recycling of the CB1 Cannabinoid Receptor. J. Neurochem. 1999, 73, 493–501.
- Hohmann, A.G.; Herkenham, M. Cannabinoid Receptors Undergo Axonal Flow in Sensory Nerves. Neuroscience 1999, 92, 1171–1175.
- Houser, S.J.; Eads, M.; Embrey, J.P.; Welch, S.P. Dynorphin B and Spinal Analgesia: Induction of Antinociception by the Cannabinoids CP55, 940, Δ9-THC and Anandamide. Brain Res. 2000, 857, 337–342.
- Beinfeld, M.C.; Connolly, K. Activation of CB1 Cannabinoid Receptors in Rat Hippocampal Slices Inhibits Potassium-Evoked Cholecystokinin Release, a Possible Mechanism Contributing to the Spatial Memory Defects Produced by Cannabinoids. Neurosci. Lett. 2001, 301, 69–71.
- Schlicker, E.; Timm, J.; Zentner, J.; Göthert, M. Cannabinoid CB1 Receptor-Mediated Inhibition of Noradrenaline Release in the Human and Guinea-Pig Hippocampus. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1997, 356, 583–589.
- Cadogan, A.; Alexander, S.P.H.; Boyd, E.A.; Kendall, D.A. Influence of Cannabinoids on Electrically Evoked Dopamine Release and Cyclic AMP Generation in the Rat Striatum. J. Neurochem. 1997, 69, 1131–1137.
- Gifford, A.N.; Ashby, C.R. Electrically Evoked Acetylcholine Release from Hippocampal Slices Is Inhibited by the Cannabinoid Receptor Agonist, WIN 55212-2, and Is Potentiated by the Cannabinoid Antagonist, SR 141716A. J. Pharmacol. Exp. Ther. 1996, 277, 1431–1436.
- Valverde, O.; Noble, F.; Beslot, F.; Daugé, V.; Fournié-Zaluski, M.; Roques, B.P. Δ9-tetrahydrocannabinol Releases and Facilitates the Effects of Endogenous Enkephalins: Reduction in Morphine Withdrawal Syndrome without Change in Rewarding Effect. Eur. J. Neurosci. 2001, 13, 1816–1824.
- Coull, M.A.; Johnston, A.T.; Pertwee, R.G.; Davies, S.N. Action of δ-9-Tetrahydrocannabinol on Gabaa Receptor-Mediated Responses in a Grease-Gap Recording Preparation of the Rat Hippocampal Slice. Neuropharmacology 1997, 36, 1387–1392.
- Maneuf, Y.P.; Nash, J.E.; Crossman, A.R.; Brotchie, J.M. Activation of the Cannabinoid Receptor by Δ9-Tetrahydrocannabinol Reduces γ-Aminobutyric Acid Uptake in the Globus Pallidus. Eur. J. Pharmacol. 1996, 308, 161–164.
- Herkenham, M.; Lynn, A.B.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Characterization and Localization of Cannabinoid Receptors in Rat Brain: A Quantitative in Vitro Autoradiographic Study. J. Neurosci. 1991, 11, 563–583.
- Hoffman, A.F.; Lupica, C.R. Mechanisms of Cannabinoid Inhibition of GABAASynaptic Transmission in the Hippocampus. J. Neurosci. 2000, 20, 2470–2479.
- Wilson, R.I.; Kunos, G.; Nicoll, R.A. Presynaptic Specificity of Endocannabinoid Signaling in the Hippocampus. Neuron 2001, 31, 453–462.
- Miller, A.S.; Walker, J.M. Effects of a Cannabinoid on Spontaneous and Evoked Neuronal Activity in the Substantia Nigra Pars Reticulata. Eur. J. Pharmacol. 1995, 279, 179–185.
- Szabo, B.; Dörner, L.; Pfreundtner, C.; Nörenberg, W.; Starke, K. Inhibition of GABAergic Inhibitory Postsynaptic Currents by Cannabinoids in Rat Corpus Striatum. Neuroscience 1998, 85, 395–403.
- Chan, P.K.Y.; Chan, S.C.Y.; Yung, W. Presynaptic Inhibition of GABAergic Inputs to Rat Substantia Nigra Pars Reticulata Neurones by a Cannabinoid Agonist. Neuroreport 1998, 9, 671–675.
- Hoffman, A.F.; Lupica, C.R. Direct Actions of Cannabinoids on Synaptic Transmission in the Nucleus Accumbens: A Comparison with Opioids. J. Neurophysiol. 2001, 85, 72–83.
- Manzoni, O.J.; Bockaert, J. Cannabinoids Inhibit GABAergic Synaptic Transmission in Mice Nucleus Accumbens. Eur. J. Pharmacol. 2001, 412, R3–R5.
- Vaughan, C.W.; McGregor, I.S.; Christie, M.J. Cannabinoid Receptor Activation Inhibits GABAergic Neurotransmission in Rostral Ventromedial Medulla Neurons in Vitro. Br. J. Pharmacol. 1999, 127, 935–940.
- Vaughan, C.W.; Connor, M.; Bagley, E.E.; Christie, M.J. Actions of Cannabinoids on Membrane Properties and Synaptic Transmission in Rat Periaqueductal Gray Neurons in Vitro. Mol. Pharmacol. 2000, 57, 288–295.
- Kreitzer, A.C.; Regehr, W.G. Cerebellar Depolarization-Induced Suppression of Inhibition Is Mediated by Endogenous Cannabinoids. J. Neurosci. 2001, 21, RC174.
- Takahashi, K.A.; Linden, D.J. Cannabinoid Receptor Modulation of Synapses Received by Cerebellar Purkinje Cells. J. Neurophysiol. 2000, 83, 1167–1180.
- Caldwell, D.; Coutts, A.A.; Mackie, K.; Irving, A.J. Cell Surface CB1 Receptors Are Expressed at Synaptic Terminals in Cultured Rat Cerebellar Granule Cells. J. Physiol.(London) 1999, 518, 152Pœ153P.
- Kreitzer, A.C.; Regehr, W.G. Retrograde Inhibition of Presynaptic Calcium Influx by Endogenous Cannabinoids at Excitatory Synapses onto Purkinje Cells. Neuron 2001, 29, 717–727.
- Maejima, T.; Ohno-Shosaku, T.; Kano, M. Endogenous Cannabinoid as a Retrograde Messenger from Depolarized Postsynaptic Neurons to Presynaptic Terminals. Neurosci. Res. 2001, 40, 205–210.
- Lévénès, C.; Daniel, H.; Soubrié, P.; Crépel, F. Cannabinoids Decrease Excitatory Synaptic Transmission and Impair Long-Term Depression in Rat Cerebellar Purkinje Cells. J. Physiol. 1998, 510, 867.
- Huang, C.; Lo, S.; Hsu, K. Presynaptic Mechanisms Underlying Cannabinoid Inhibition of Excitatory Synaptic Transmission in Rat Striatal Neurons. J. Physiol. 2001, 532, 731–748.
- Robbe, D.; Alonso, G.; Duchamp, F.; Bockaert, J.; Manzoni, O.J. Localization and Mechanisms of Action of Cannabinoid Receptors at the Glutamatergic Synapses of the Mouse Nucleus Accumbens. J. Neurosci. 2001, 21, 109–116.
- Shen, M.; Piser, T.M.; Seybold, V.S.; Thayer, S.A. Cannabinoid Receptor Agonists Inhibit Glutamatergic Synaptic Transmission in Rat Hippocampal Cultures. J. Neurosci. 1996, 16, 4322–4334.
- Misner, D.L.; Sullivan, J.M. Mechanism of Cannabinoid Effects on Long-Term Potentiation and Depression in Hippocampal CA1 Neurons. J. Neurosci. 1999, 19, 6795–6805.
- Paton, G.S.; Pertwee, R.G.; Davies, S.N. Correlation between Cannabinoid Mediated Effects on Paired Pulse Depression and Induction of Long Term Potentiation in the Rat Hippocampal Slice. Neuropharmacology 1998, 37, 1123–1130.
- Collins, D.R.; Pertwee, R.G.; Davies, S.N. The Action of Synthetic Cannabinoids on the Induction of Long-Term Potentiation in the Rat Hippocampal Slice. Eur. J. Pharmacol. 1994, 259, R7–R8.
- Terranova, J.P.; Michaud, J.C.; Fur, G.L.; Soubrié, P. Inhibition of Long-Term Potentiation in Rat Hippocampal Slices by Anandamide and WIN55212-2: Reversal by SR141716 A, a Selective Antagonist of CB1 Cannabinoid Receptors. Naunyn Schmiedebergs Arch Pharmacol. 1995, 352, 576–579.
- Auclair, N.; Otani, S.; Soubrie, P.; Crepel, F. Cannabinoids Modulate Synaptic Strength and Plasticity at Glutamatergic Synapses of Rat Prefrontal Cortex Pyramidal Neurons. J. Neurophysiol. 2000, 83, 3287–3293.
- Gerdeman, G.; Lovinger, D.M. CB1 Cannabinoid Receptor Inhibits Synaptic Release of Glutamate in Rat Dorsolateral Striatum. J. Neurophysiol. 2001, 85, 468–471.
- Lazenka, M.F.; Selley, D.E.; Sim-Selley, L.J. Brain Regional Differences in CB1 Receptor Adaptation and Regulation of Transcription. Life Sci. 2013, 92, 446–452.
- Sim-Selley, L.J. Regulation of Cannabinoid CB1 Receptors in the Central Nervous System by Chronic Cannabinoids. Crit. Rev. Neurobiol. 2003, 15, 30.
- Villares, J. Chronic Use of Marijuana Decreases Cannabinoid Receptor Binding and MRNA Expression in the Human Brain. Neuroscience 2007, 145, 323–334.
- Hirvonen, J.; Goodwin, R.S.; Li, C.-T.; Terry, G.E.; Zoghbi, S.S.; Morse, C.; Pike, V.W.; Volkow, N.D.; Huestis, M.A.; Innis, R. Reversible and Regionally Selective Downregulation of Brain Cannabinoid CB1 Receptors in Chronic Daily Cannabis Smokers. Mol. Psychiatry 2012, 17, 642–649.
- Herdegen, T.; Leah, J.D. Inducible and Constitutive Transcription Factors in the Mammalian Nervous System: Control of Gene Expression by Jun, Fos and Krox, and CREB/ATF Proteins. Brain Res. Rev. 1998, 28, 370–490.
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol. Rev. 2002, 54, 161–202.
- Kasukawa, T.; Masumoto, K.; Nikaido, I.; Nagano, M.; Uno, K.D.; Tsujino, K.; Hanashima, C.; Shigeyoshi, Y.; Ueda, H.R. Quantitative Expression Profile of Distinct Functional Regions in the Adult Mouse Brain. PLoS ONE 2011, 6, e23228.
- Aymerich, M.S.; Aso, E.; Abellanas, M.A.; Tolon, R.M.; Ramos, J.A.; Ferrer, I.; Romero, J.; Fernández-Ruiz, J. Cannabinoid Pharmacology/Therapeutics in Chronic Degenerative Disorders Affecting the Central Nervous System. Biochem. Pharmacol. 2018, 157, 67–84.
- Cassano, T.; Calcagnini, S.; Pace, L.; De Marco, F.; Romano, A.; Gaetani, S. Cannabinoid Receptor 2 Signaling in Neurodegenerative Disorders: From Pathogenesis to a Promising Therapeutic Target. Front. Neurosci. 2017, 11, 30.
- Chung, Y.C.; Shin, W.-H.; Baek, J.Y.; Cho, E.J.; Baik, H.H.; Kim, S.R.; Won, S.-Y.; Jin, B.K. CB2 Receptor Activation Prevents Glial-Derived Neurotoxic Mediator Production, BBB Leakage and Peripheral Immune Cell Infiltration and Rescues Dopamine Neurons in the MPTP Model of Parkinson’s Disease. Exp. Mol. Med. 2016, 48, e205.
- Palazuelos, J.; Ortega, Z.; Díaz-Alonso, J.; Guzmán, M.; Galve-Roperh, I. CB2 Cannabinoid Receptors Promote Neural Progenitor Cell Proliferation via MTORC1 Signaling. J. Biol. Chem. 2012, 287, 1198–1209.
- Burdyga, G.; Lal, S.; Varro, A.; Dimaline, R.; Thompson, D.G.; Dockray, G.J. Expression of Cannabinoid CB1 Receptors by Vagal Afferent Neurons Is Inhibited by Cholecystokinin. J. Neurosci. 2004, 24, 2708–2715.
- McCoy, K.L.; Matveyeva, M.; Carlisle, S.J.; Cabral, G.A. Cannabinoid Inhibition of the Processing of Intact Lysozyme by Macrophages: Evidence for CB2 Receptor Participation. J. Pharmacol. Exp. Ther. 1999, 289, 1620–1625.
- Schatz, A.R.; Lee, M.; Condie, R.B.; Pulaski, J.T.; Kaminski, N.E. Cannabinoid Receptors CB1 and CB2: A Characterization of Expression and Adenylate Cyclase Modulation within the Immune System. Toxicol. Appl. Pharmacol. 1997, 142, 278–287.
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; LE Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61.
- Buckley, N.E.; McCoy, K.L.; Mezey, É.; Bonner, T.; Zimmer, A.; Felder, C.C.; Glass, M.; Zimmer, A. Immunomodulation by Cannabinoids Is Absent in Mice Deficient for the Cannabinoid CB2 Receptor. Eur. J. Pharmacol. 2000, 396, 141–149.
- Buckley, N.E. The Peripheral Cannabinoid Receptor Knockout Mice: An Update. Br. J. Pharmacol. 2008, 153, 309–318.
- Miller, L.K.; Devi, L.A. The Highs and Lows of Cannabinoid Receptor Expression in Disease: Mechanisms and Their Therapeutic Implications. Pharmacol. Rev. 2011, 63, 461–470.
- Onaivi, E.S.; Ishiguro, H.; Gong, J.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E. Functional Expression of Brain Neuronal CB2 Cannabinoid Receptors Are Involved in the Effects of Drugs of Abuse and in Depression. Ann. N. Y. Acad. Sci. 2008, 1139, 434–449.
- Onaivi, E.S.; Ishiguro, H.; Gu, S.; Liu, Q.-R. CNS Effects of CB2 Cannabinoid Receptors: Beyond Neuro-Immuno-Cannabinoid Activity. J. Psychopharmacol. 2012, 26, 92–103.
- Ma, Z.; Gao, F.; Larsen, B.; Gao, M.; Luo, Z.; Chen, D.; Ma, X.; Qiu, S.; Zhou, Y.; Xie, J.; et al. Mechanisms of Cannabinoid CB(2) Receptor-Mediated Reduction of Dopamine Neuronal Excitability in Mouse Ventral Tegmental Area. EBioMedicine 2019, 42, 225–237.
- Rathod, S.S.; Agrawal, Y.O. Phytocannabinoids as Potential Multitargeting Neuroprotectants in Alzheimer’s Disease. Curr. Drug Res. Rev. 2023.
- Xin, Q.; Xu, F.; Taylor, D.H.; Zhao, J.-F.; Wu, J. The Impact of Cannabinoid Type 2 Receptors (CB2Rs) in Neuroprotection against Neurological Disorders. Acta Pharmacol. Sin. 2020, 41, 1507–1518.
- Zhang, H.-Y.; Gao, M.; Liu, Q.-R.; Bi, G.-H.; Li, X.; Yang, H.-J.; Gardner, E.L.; Wu, J.; Xi, Z.-X. Cannabinoid CB2 Receptors Modulate Midbrain Dopamine Neuronal Activity and Dopamine-Related Behavior in Mice. Proc. Natl. Acad. Sci. USA 2014, 111, E5007–E5015.
- Stempel, A.V.; Stumpf, A.; Zhang, H.-Y.; Özdoğan, T.; Pannasch, U.; Theis, A.-K.; Otte, D.-M.; Wojtalla, A.; Rácz, I.; Ponomarenko, A. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 2016, 90, 795–809.
- den Boon, F.S.; Chameau, P.; Schaafsma-Zhao, Q.; van Aken, W.; Bari, M.; Oddi, S.; Kruse, C.G.; Maccarrone, M.; Wadman, W.J.; Werkman, T.R. Excitability of Prefrontal Cortical Pyramidal Neurons Is Modulated by Activation of Intracellular Type-2 Cannabinoid Receptors. Proc. Natl. Acad. Sci. USA. 2012, 109, 3534–3539.
- Vlachou, S.; Panagis, G. Regulation of Brain Reward by the Endocannabinoid System: A Critical Review of Behavioral Studies in Animals. Curr. Pharm. Des. 2014, 20, 2072–2088.
- Agudo, J.; Martin, M.; Roca, C.; Molas, M.; Bura, A.S.; Zimmer, A.; Bosch, F.; Maldonado, R. Deficiency of CB2 Cannabinoid Receptor in Mice Improves Insulin Sensitivity but Increases Food Intake and Obesity with Age. Diabetologia 2010, 53, 2629–2640.
- Ignatowska-Jankowska, B.; Jankowski, M.M.; Swiergiel, A.H. Cannabidiol Decreases Body Weight Gain in Rats: Involvement of CB2 Receptors. Neurosci. Lett. 2011, 490, 82–84.
- Flake, N.M.; Zweifel, L.S. Behavioral Effects of Pulp Exposure in Mice Lacking Cannabinoid Receptor 2. J. Endod. 2012, 38, 86–90.
- Emadi, L.; Jonaidi, H.; Hosseini Amir Abad, E. The Role of Central CB2 Cannabinoid Receptors on Food Intake in Neonatal Chicks. J. Comp. Physiol. A 2011, 197, 1143–1147.
- García-Gutiérrez, M.S.; Pérez-Ortiz, J.M.; Gutiérrez-Adán, A.; Manzanares, J. Depression-resistant Endophenotype in Mice Overexpressing Cannabinoid CB2 Receptors. Br. J. Pharmacol. 2010, 160, 1773–1784.
- García-Gutiérrez, M.S.; Manzanares, J. Overexpression of CB2 Cannabinoid Receptors Decreased Vulnerability to Anxiety and Impaired Anxiolytic Action of Alprazolam in Mice. J. Psychopharmacol. 2011, 25, 111–120.
- Ortega-Alvaro, A.; Aracil-Fernández, A.; García-Gutiérrez, M.S.; Navarrete, F.; Manzanares, J. Deletion of CB2 Cannabinoid Receptor Induces Schizophrenia-Related Behaviors in Mice. Neuropsychopharmacology 2011, 36, 1489–1504.
- Xi, Z.-X.; Peng, X.-Q.; Li, X.; Song, R.; Zhang, H.-Y.; Liu, Q.-R.; Yang, H.-J.; Bi, G.-H.; Li, J.; Gardner, E.L. Brain Cannabinoid CB2 Receptors Modulate Cocaine’s Actions in Mice. Nat. Neurosci. 2011, 14, 1160–1166.
- Ortega-Álvaro, A.; Ternianov, A.; Aracil-Fernández, A.; Navarrete, F.; García-Gutiérrez, M.S.; Manzanares, J. Role of Cannabinoid CB2 Receptor in the Reinforcing Actions of Ethanol. Addict. Biol. 2015, 20, 43–55.
- Navarrete, F.; Rodríguez-Arias, M.; Martín-García, E.; Navarro, D.; García-Gutiérrez, M.S.; Aguilar, M.A.; Aracil-Fernández, A.; Berbel, P.; Miñarro, J.; Maldonado, R. Role of CB2 Cannabinoid Receptors in the Rewarding, Reinforcing, and Physical Effects of Nicotine. Neuropsychopharmacology 2013, 38, 2515–2524.
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65.
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E. Peripheral Cannabinoid Receptor, CB2, Regulates Bone Mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701.
- Benito, C.; Núñez, E.; Tolón, R.M.; Carrier, E.J.; Rábano, A.; Hillard, C.J.; Romero, J. Cannabinoid CB2 Receptors and Fatty Acid Amide Hydrolase Are Selectively Overexpressed in Neuritic Plaque-Associated Glia in Alzheimer’s Disease Brains. J. Neurosci. 2003, 23, 11136–11141.
- Benito, C.; Kim, W.-K.; Chavarría, I.; Hillard, C.J.; Mackie, K.; Tolón, R.M.; Williams, K.; Romero, J. A Glial Endogenous Cannabinoid System Is Upregulated in the Brains of Macaques with Simian Immunodeficiency Virus-Induced Encephalitis. J. Neurosci. 2005, 25, 2530–2536.
- Ramírez, B.G.; Blázquez, C.; Del Pulgar, T.G.; Guzmán, M.; de Ceballos, M.L. Prevention of Alzheimer’s Disease Pathology by Cannabinoids: Neuroprotection Mediated by Blockade of Microglial Activation. J. Neurosci. 2005, 25, 1904–1913.
- Ashton, J.C.; Rahman, R.M.A.; Nair, S.M.; Sutherland, B.A.; Glass, M.; Appleton, I. Cerebral Hypoxia-Ischemia and Middle Cerebral Artery Occlusion Induce Expression of the Cannabinoid CB2 Receptor in the Brain. Neurosci. Lett. 2007, 412, 114–117.
- Naguib, M.; Xu, J.J.; Diaz, P.; Brown, D.L.; Cogdell, D.; Bie, B.; Hu, J.; Craig, S.; Hittelman, W.N. Prevention of Paclitaxel-Induced Neuropathy through Activation of the Central Cannabinoid Type 2 Receptor System. Anesth. Analg. 2012, 114, 1104.
- Wu, J.; Bie, B.; Yang, H.; Xu, J.J.; Brown, D.L.; Naguib, M. Activation of the CB2 Receptor System Reverses Amyloid-Induced Memory Deficiency. Neurobiol. Aging 2013, 34, 791–804.
- Wu, J.; Hocevar, M.; Foss, J.F.; Bie, B.; Naguib, M. Activation of CB2 Receptor System Restores Cognitive Capacity and Hippocampal Sox2 Expression in a Transgenic Mouse Model of Alzheimer’s Disease. Eur. J. Pharmacol. 2017, 811, 12–20.
- Xu, J.; Tang, Y.; Xie, M.; Bie, B.; Wu, J.; Yang, H.; Foss, J.F.; Yang, B.; Rosenquist, R.W.; Naguib, M. Activation of Cannabinoid Receptor 2 Attenuates Mechanical Allodynia and Neuroinflammatory Responses in a Chronic Post-ischemic Pain Model of Complex Regional Pain Syndrome Type I in Rats. Eur. J. Neurosci. 2016, 44, 3046–3055.
- Romero-Sandoval, E.A.; Horvath, R.J.; DeLeo, J.A. Neuroimmune Interactions and Pain: Focus on Glial-Modulating Targets. Curr. Opin. Investig. drugs 2008, 9, 726.
- Villacampa, N.; Heneka, M.T. Microglia: You’ll Never Walk Alone! Immunity 2018, 48, 195–197.
- Zhang, J.; Hoffert, C.; Vu, H.K.; Groblewski, T.; Ahmad, S.; O’Donnell, D. Induction of CB2 Receptor Expression in the Rat Spinal Cord of Neuropathic but Not Inflammatory Chronic Pain Models. Eur. J. Neurosci. 2003, 17, 2750–2754.
- Schafer, D.P.; Stevens, B. Phagocytic Glial Cells: Sculpting Synaptic Circuits in the Developing Nervous System. Curr. Opin. Neurobiol. 2013, 23, 1034–1040.
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705.
- Tremblay, M.-È.; Majewska, A.K. A Role for Microglia in Synaptic Plasticity? Commun. Integr. Biol. 2011, 4, 220–222.
- Tremblay, M.-È.; Lowery, R.L.; Majewska, A.K. Microglial Interactions with Synapses Are Modulated by Visual Experience. PLoS Biol. 2010, 8, e1000527.
- Zhang, W.; Lu, L.; Lai, Q.; Zhu, B.; Li, Z.; Xu, Y.; Shao, Z.; Herrup, K.; Moore, B.S.; Ross, A.C. Family-Wide Structural Characterization and Genomic Comparisons Decode the Diversity-Oriented Biosynthesis of Thalassospiramides by Marine Proteobacteria. J. Biol. Chem. 2016, 291, 27228–27238.
- Wotherspoon, G.; Fox, A.; McIntyre, P.; Colley, S.; Bevan, S.; Winter, J. Peripheral Nerve Injury Induces Cannabinoid Receptor 2 Protein Expression in Rat Sensory Neurons. Neuroscience 2005, 135, 235–245.
- Romero-Sandoval, A.; Nutile-McMenemy, N.; DeLeo, J.A. Spinal Microglial and Perivascular Cell Cannabinoid Receptor Type 2 Activation Reduces Behavioral Hypersensitivity without Tolerance after Peripheral Nerve Injury. J. Am. Soc. Anesthesiol. 2008, 108, 722–734.
- Svíženská, I.H.; Brázda, V.; Klusáková, I.; Dubový, P. Bilateral Changes of Cannabinoid Receptor Type 2 Protein and MRNA in the Dorsal Root Ganglia of a Rat Neuropathic Pain Model. J. Histochem. Cytochem. 2013, 61, 529–547.
- Solas, M.; Francis, P.T.; Franco, R.; Ramirez, M.J. CB2 Receptor and Amyloid Pathology in Frontal Cortex of Alzheimer’s Disease Patients. Neurobiol. Aging 2013, 34, 805–808.
- Tolón, R.M.; Núñez, E.; Pazos, M.R.; Benito, C.; Castillo, A.I.; Martínez-Orgado, J.A.; Romero, J. The Activation of Cannabinoid CB2 Receptors Stimulates in Situ and in Vitro Beta-Amyloid Removal by Human Macrophages. Brain Res. 2009, 1283, 148–154.
- Zarruk, J.G.; Fernández-López, D.; García-Yébenes, I.; García-Gutiérrez, M.S.; Vivancos, J.; Nombela, F.; Torres, M.; Burguete, M.C.; Manzanares, J.; Lizasoain, I. Cannabinoid Type 2 Receptor Activation Downregulates Stroke-Induced Classic and Alternative Brain Macrophage/Microglial Activation Concomitant to Neuroprotection. Stroke 2012, 43, 211–219.
- Sun, H.; Gong, S.; Carmody, R.J.; Hilliard, A.; Li, L.; Sun, J.; Kong, L.; Xu, L.; Hilliard, B.; Hu, S. TIPE2, a Negative Regulator of Innate and Adaptive Immunity That Maintains Immune Homeostasis. Cell 2008, 133, 415–426.
- Ertl, N.G.; O’Connor, W.A.; Papanicolaou, A.; Wiegand, A.N.; Elizur, A. Transcriptome Analysis of the Sydney Rock Oyster, Saccostrea Glomerata: Insights into Molluscan Immunity. PLoS ONE 2016, 11, e0156649.
- Hou, S.T.; Jiang, S.X.; Smith, R.A. Permissive and Repulsive Cues and Signalling Pathways of Axonal Outgrowth and Regeneration. Int. Rev. Cell Mol. Biol. 2008, 267, 125–181.
- Fernandez-Ruiz, J.; Romero, J.; Velasco, G.; Tolon, R.M.; Ramos, J.A.; Guzman, M. Cannabinoid CB2 Receptor: A New Target for Controlling Neural Cell Survival? Trends Pharmacol. Sci. 2007, 28, 39–45.
- Lotersztajn, S.; Teixeira-Clerc, F.; Julien, B.; Deveaux, V.; Ichigotani, Y.; Manin, S.; Tran-Van-Nhieu, J.; Karsak, M.; Zimmer, A.; Mallat, A. CB2 Receptors as New Therapeutic Targets for Liver Diseases. Br. J. Pharmacol. 2008, 153, 286–289.
- Wright, K.L.; Duncan, M.; Sharkey, K.A. Cannabinoid CB2 Receptors in the Gastrointestinal Tract: A Regulatory System in States of Inflammation. Br. J. Pharmacol. 2008, 153, 263–270.
- Merighi, S.; Gessi, S.; Varani, K.; Fazzi, D.; Mirandola, P.; Borea, P.A. Cannabinoid CB2 Receptor Attenuates Morphine-induced Inflammatory Responses in Activated Microglial Cells. Br. J. Pharmacol. 2012, 166, 2371–2385.
- Arévalo-Martín, A.; García-Ovejero, D.; Gomez, O.; Rubio-Araiz, A.; Navarro-Galve, B.; Guaza, C.; Molina-Holgado, E.; Molina-Holgado, F. CB2 Cannabinoid Receptors as an Emerging Target for Demyelinating Diseases: From Neuroimmune Interactions to Cell Replacement Strategies. Br. J. Pharmacol. 2008, 153, 216–225.
- Romero-Sandoval, A.; Eisenach, J.C. Spinal Cannabinoid Receptor Type 2 Activation Reduces Hypersensitivity and Spinal Cord Glial Activation after Paw Incision. J. Am. Soc. Anesthesiol. 2007, 106, 787–794.
- Eljaschewitsch, E.; Witting, A.; Mawrin, C.; Lee, T.; Schmidt, P.M.; Wolf, S.; Hoertnagl, H.; Raine, C.S.; Schneider-Stock, R.; Nitsch, R. The Endocannabinoid Anandamide Protects Neurons during CNS Inflammation by Induction of MKP-1 in Microglial Cells. Neuron 2006, 49, 67–79.
- Martin, M.; Michalek, S.M.; Katz, J. Role of Innate Immune Factors in the Adjuvant Activity of Monophosphoryl Lipid A. Infect. Immun. 2003, 71, 2498–2507.
- Diaz, P.; Phatak, S.S.; Xu, J.; Fronczek, F.R.; Astruc-Diaz, F.; Thompson, C.M.; Cavasotto, C.N.; Naguib, M. 2, 3-Dihydro-1-Benzofuran Derivatives as a Series of Potent Selective Cannabinoid Receptor 2 Agonists: Design, Synthesis, and Binding Mode Prediction through Ligand-Steered Modeling. ChemMedChem Chem. Enabling Drug Discov. 2009, 4, 1615–1629.
- Xu, J.J.; Diaz, P.; Bie, B.; Astruc-Diaz, F.; Wu, J.; Yang, H.; Brown, D.L.; Naguib, M. Spinal Gene Expression Profiling and Pathways Analysis of a CB2 Agonist (MDA7)-Targeted Prevention of Paclitaxel-Induced Neuropathy. Neuroscience 2014, 260, 185–194.
- Gong, J.-P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.-R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 Receptors: Immunohistochemical Localization in Rat Brain. Brain Res. 2006, 1071, 10–23.
- Ashton, J.C.; Friberg, D.; Darlington, C.L.; Smith, P.F. Expression of the Cannabinoid CB2 Receptor in the Rat Cerebellum: An Immunohistochemical Study. Neurosci. Lett. 2006, 396, 113–116.
- Beltramo, M.; Bernardini, N.; Bertorelli, R.; Campanella, M.; Nicolussi, E.; Fredduzzi, S.; Reggiani, A. CB2 Receptor-mediated Antihyperalgesia: Possible Direct Involvement of Neural Mechanisms. Eur. J. Neurosci. 2006, 23, 1530–1538.
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the Cannabinoid CB2 Receptor in Microglial Cells in Response to Inflammatory Stimuli. J. Neurochem. 2005, 95, 437–445.
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332.
- Derbenev, A.V.; Stuart, T.C.; Smith, B.N. Cannabinoids Suppress Synaptic Input to Neurones of the Rat Dorsal Motor Nucleus of the Vagus Nerve. J. Physiol. 2004, 559, 923–938.
- Pamplona, F.A.; Prediger, R.D.S.; Pandolfo, P.; Takahashi, R.N. The Cannabinoid Receptor Agonist WIN 55,212-2 Facilitates the Extinction of Contextual Fear Memory and Spatial Memory in Rats. Psychopharmacology 2006, 188, 641–649.
- Reggio, P.H. Endocannabinoid Binding to the Cannabinoid Receptors: What Is Known and What Remains Unknown. Curr. Med. Chem. 2010, 17, 1468–1486.
- Atwood, B.K.; Mackie, K. CB2: A Cannabinoid Receptor with an Identity Crisis. Br. J. Pharmacol. 2010, 160, 467–479.
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064.
- Onaivi, E.S.; Fantini, N.; Carai, M.A.M.; Gessa, G.L.; Colombo, G. CNS Effects of CB2 Cannabinoid Receptors. Open Neuropsychopharmacol. J. 2009, 2.
- Kivrak, B.G.; Erzurumlu, R.S. Development of the Principal Nucleus Trigeminal Lemniscal Projections in the Mouse. J. Comp. Neurol. 2013, 521, 299–311.
