Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Gastroenterology & Hepatology
Irritable bowel syndrome (IBS) is a severe problem in the health service. It is estimated that it accounts for 3% of all medical consultations. Moreover, this diagnosis is made in the case of about 40% of all outpatient gastroenterological referrals. The visceral stimuli from the digestive tract are transmitted via afferent nerves through the spinal cord to the brain, where they are felt as pain. The overreaction observed in the brain of irritable bowel syndrome (IBS) patients may be due to increased peripheral sensitivity to stimuli from the gastrointestinal tract.
- visceral hypersensitivity
- irritable bowel syndrome
- vagus nerve
- nutraceuticals
1. The Role of the Vagus Nerve as a Modulator of the Brain–Gut Axis
“My function’s almost anything, and vagus is my name” [27].
The vagus nerve (VN), also called the “great wandering protector” [28], is part of the parasympathetic nervous system, is the longest cranial nerve, and is a crucial bidirectional channel between the body and the brain, mainly for maintaining homeostasis [29]. The vagus nerve is essentially a sensory nerve that informs the brain of the state of visceral organs, such as the intestines [30].
All sensory information converges in the nuclei of the VN, which is then transmitted to many brain areas. The relevant regulatory information is transmitted from these areas by the descending efferents of the vagus nerve [28].
The balance between the activity of the sympathetic and parasympathetic systems is referred to as the sympathetic–vagal balance, which is involved in regulating homeostasis. Disruption of this balance is conducive to but also reflects pathological conditions [30].
From a physiological point of view, the area of parasympathetic preganglionic neurons modulates the functions of the digestive system. It consists of afferent fibers of the vagus nerve, nucleus tractus solitarius (NTS) neurons located in the medulla oblongata, and efferent fibers originating from the dorsal motor nucleus of the vagus nerve (DMV) within the brainstem. Neural communication between the NTS and the DMV is vital. Research indicates that the presence of various neurotransmitter hormones regulates it, but also the presence or absence of transmission afferent input to the NTS [31].
VN plays a crucial role in determining brain–body interactions. Vagal activity tone is positively influenced by health, relaxation, well-being, and emotions such as empathy, while it is negatively influenced by risk factors such as morbidity and stress [27].
The multiplicity of functions of the VN causes an increase in the interest of scientists in artificial stimulation of the vagus nerve for therapeutic purposes [27].
The research indicates a relationship between vagus nerve stimulation (VNS) and the activation of neuromodulatory networks in the central nervous system. Manual or electrical techniques are used for vagal nerve stimulation [32]. The long signals of the delivered VNS increase neuronal activity in the dorsal raphe nucleus and locus coeruleus. Moreover, to change after intense stimulation, VNS provides rapid activation of neuromodulatory pathways. Vagus nerve stimulation causes immediate changes in cortical synchronization, but the effect depends on the activation of muscarinic acetylcholine receptors [33]. Muscarinic acetylcholine receptors have been divided genetically and pharmacologically into five subtypes (M1–M5). Smooth muscle tissues show heterogeneous expression of the M2 and M3 subtypes with a population of approximately 4:1 and play an essential role in intestinal contractile responses [34]. Studies indicate that IBS patients display an exaggerated muscarinic-receptor-mediated IL-6 response [35].
Chen et al. studied the effects of vagal nerve stimulation (VNS) on CRF-induced changes in serum ACTH levels in rats. The conducted analyses showed that 2 h of continuous vagus nerve stimulation inhibited CRF-induced ACTH release compared to the control group [36]. Similarly, research by Nijsen et al. suggests that endogenous CRH reduces the vagal response to conditioned fear stress in rats [37].
A study by Pellissier et al. showed that high morning vagal tone is associated with low evening cortisol levels in healthy individuals. Unfortunately, the demonstrated correlations are not observed in IBS patients, suggesting a disorder between vagal tone and cortisol levels in these patients. Additionally, research indicates that IBS patients with low vagal tone exhibit high plasma epinephrine levels as a sign of maladjusted high sympathetic activity [38].
It has been shown that stress stimulates the sympathetic nervous system while inhibiting VN [30]. There is a close relationship between exposure to stress and developing IBS symptoms, which are influenced by both autonomic and neuroendocrine responses. Studies have shown that people who experience severe stress reactions in childhood are more likely to develop IBS later in life, showing a strong link between the brain and the digestive system [39]. The consequence of reducing the activity of the vagus nerve, for example, as a result of stress, is the increased secretion and permeability of the intestines and the intensification of inflammation in the intestines. In addition, pain receptors become more sensitive, which increases the perception of visceral pain in IBS [40]. Incorrect vagus nerve tension is described in IBS and inflammatory bowel disease (IBD), which is why it seems reasonable to state that VN (VNS) stimulation may become an effective therapy in the treatment of these diseases [30].
VNS connects specific peripheral sensors and effectors to the central nervous system. VN-mediated connections involve projections to the hypothalamus and cortex as higher brain regions, thus allowing VN modulatory access to subcortical and cortical brain regions. Thus, the signals generated in the VN can affect a wide range of basic brain functions and the whole organism’s protection (Figure 1) [33].
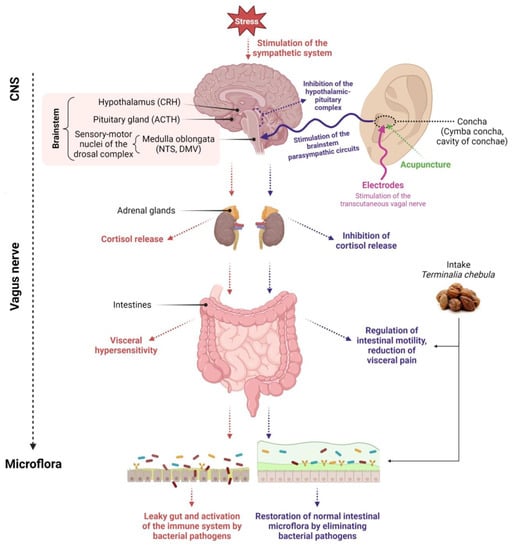
Figure 1. Diagram depicting the influence of various clinical methods on the restoration of microflora. Created with BioRender.com, accessed on 1 September 2023.
The external ear is the only place on the body where VN sends its only peripheral branch. The auricular branch of VN surfaces, as the afferent auricular VN (aVN), is susceptible to external stimuli in terms of peripheral nerve stimulation. It allows for easy external access via electrical stimulation in terms of aVNS, which then connects the applied stimuli to the brainstem. Thus, the auricle, especially its aVN endings, might become a critical area of the body to modulate various brain functions, offering non-invasive manipulation of the central nervous system via the vagus nerve [33].
Therefore, transcutaneous VNS (tVNS) is a very interesting therapy form. It is a non-invasive method that stimulates the afferent nerve fibers of the vagus nerve in the ear. According to the “bottom-up” mechanism of the CNS, electrical stimulation of these areas can cause changes in the activity of the VN pathway in the central structures and the brainstem. taVNS (transcutaneous auricular VNS) has been successfully used to treat disorders such as depression, epilepsy, chronic tinnitus, or pre-diabetes [41].
In recent years, transcutaneous auricular VNS (taVNS) has been reported to alleviate gastrointestinal disorders. A study of forty-two IBS-C patients randomized to a 4-week sham treatment with taVNS or taVNS showed that non-invasive taVNS reduced constipation and abdominal pain in IBS-C patients. Moreover, the applied taVNS therapy reduced serum TNF-α and IL-6 levels and plasma 5-HT levels. The improvement in IBS-C symptoms was justified by the integrative effect of taVNS on gut function mediated by autoimmune mechanisms [42]. Clinical data on the use of taVNS in treating conditions such as depression and pain suggest that it is safe and well tolerated [32].
Both neural communication via the vagus nerve and hormonal communication via the hypothalamic–pituitary–adrenal axis (HPA axis) together enable the brain to influence the activity of functional effector cells of the intestine, such as epithelial cells, immune cells, intestinal neurons, smooth muscle cells, and enterochromaffin cells. It turns out that these cells are under the influence of the intestinal microflora. The gut microbiota also significantly impacts the brain–gut axis, locally interacting with intestinal cells and the enteric nervous system (ENS) and directly affecting the metabolic and neuroendocrine systems [43].
Acupuncture is also a form of therapy for IBS. It is a traditional Chinese medical practice that is increasingly accepted and used in Western society. Acupuncture, widely used in IBS, involves stimulation of the vagus nerve and, thus, stimulation of the somatic nervous system. Studies show that the applied therapy can alleviate visceral hypersensitivity and improve intestinal motility in patients with IBS [44].
A multicenter, randomized, controlled trial involving 531 patients diagnosed with IBS showed that acupuncture was more effective than pinaverium bromide in relieving IBS symptoms and improving quality of life during a 1.5-month treatment and a subsequent three-month follow-up period [45].
2. The Vagus Nerve Can Recognize the Microflora and Provide This Information to the Central Nervous System
Changes in the composition of the intestinal flora are important in developing intestinal diseases. Unfortunately, the mechanisms leading to the development of the disease process remain largely undefined. In this case, the activity of signaling molecules and the recognition of bacterial epitopes by immune cells of both the intestinal epithelium and the mucosa may play an important role [46].
Stress can modify the intestinal microflora by releasing stress hormones, while dysbiosis is observed in both IBS and IBD. Communication between the brain and the microflora is two-way, through many trials: nervous via VN and/or spinal cord, hormonal, immunological, and metabolic [30].
Dysbiosis of the intestinal microbiota composition may cause intestinal permeability, intestinal motility disorders, visceral hypersensitivity, and anxiety disorders, or depression [47].
Commensal gut bacteria and gut pathogens can also alter and modulate visceral hypersensitivity to pain. The microbiota mediates visceral nociception by regulating visceral afferents either directly or through interfering with gut barrier function and the gut immune system [48]. In preclinical studies in animal models, the gut microbiota has been found to play a crucial role in hypersensitivity. For example, antibiotics administered early in life have been shown to cause long-term exacerbation of visceral pain in adult mice by disrupting the gut microbiota. In contrast, studies in rats showed that treatment with vancomycin early in life also increased visceral pain caused by distension of the large intestine. Other preclinical studies have also provided evidence that probiotics can regulate visceral pain. Data from studies in microbe-free mice showed that the gut microbiota has a decisive influence on visceral pain, as germ-free mice exhibited visceral hypersensitivity accompanied by upregulation of Toll-like receptors and cytokines in the spinal cord.
In contrast, hypersensitivity was abolished through colonization by postnatal microflora from conventionally colonized animals [49]. A clinical trial study of 362 IBS women showed that B. infantis effectively reduces pain and abdominal bloating and improves intestinal movements after four weeks of treatment compared to placebo. Similarly, the use of L. rhamnosus and L. plantarum in patients with IBS showed reduced pain and bloating in two studies. In another study, where patients were administered probiotics for eight weeks, abdominal pain was also reduced [48].
Improper fermentation can be an important factor in the development of diseases of the digestive tract. Intestinal infections or antibiotic therapy can damage the colonic microflora, increasing fermentation and gas accumulation. Intestinal gas is associated with symptoms such as bloating, discomfort, constipation, belching, and abdominal pain. In addition, they are among the most common health complaints that cause people to visit a gastroenterologist [50].
Sulfate-reducing bacteria (SRB) present in the human colon dissimilate sulfate to hydrogen sulfide in a process called sulfate dissimilation reduction. Hydrogen sulfide is highly toxic not only to the host but also to the bacteria that produce it. High concentrations of hydrogen sulfide lead to the inhibition of SRB growth and the development of inflammatory damage to the intestinal epithelium [51].
A diet rich in sulfate ions, such as foods preserved with sulfur oxides, increases the hydrogen sulfide concentration produced by SRBs and can lead to gastrointestinal diseases. Research reports that the products included in the Western diet contain as much as 16.6 mmol of sulfates per day [51].
SRB can exacerbate gastrointestinal disease by producing the toxic product hydrogen sulfide and reducing the production of beneficial butyrate, the preferred energy source for colonocytes [52]. Butyrate has a normalizing effect on functional disorders of the intestines and has anti-inflammatory properties in the large intestine [38]. In addition, some SRB species may be associated with gastrointestinal diseases. The incidence of Desulfovibrio piger was significantly higher in patients with inflammatory bowel disease compared to healthy controls [52]. In addition, Desulfovibrio metabolites are also crucial factors leading to the formation and development of intestinal diseases, stimulating the body to release inflammatory factors such as Interleukin-6 or Interleukin-8. One of the primary metabolites of Desulfovibrio is the hydrogen sulfide mentioned above, which is cytotoxic in high concentrations [53].
The intestinal microflora also contains methanogenic archaea.
Methanogens are essential components of the intestinal microbiota colonizing the intestines, a group of microorganisms classified as archaea. Archaea produce methane by utilizing molecular hydrogen and carbon dioxide during the oxidation of organic acids [54].
Several lines of evidence indicate that archaea are absent in infancy while ubiquitous in school-aged children, suggesting that colonization is due to childhood environmental exposure [55].
Anaerobic methanogens are the primary sources of methane in the intestines. M. smithii, isolated from various phylotypes, is involved in over 90% of methane production and constitutes 10% of the intestinal microbiome [56].
It has been shown that IBS-C patients have significantly different gut microbiota compared to IBS-D patients. The gut microbiome of IBS-C individuals has more archaeal methanogens than IBS-D, which produce methane by fermenting endogenous and exogenous carbohydrates [56]. The association of irritable bowel syndrome with methane makes it the center of attention and further underscores the importance of studying this relationship for disease symptoms and possible treatments for IBS.
Kim et al. investigated the importance of M. smithii as a determinant of methane production in IBS patients with detectable methane on a breath test and in patients without detectable methane. The studies were conducted in stool samples using quantitative polymerase chain reaction. It has been shown that M. smithii is the significant methanogen responsible for respiratory methane in people with IBS. In addition, the level of M. smithii in fecal samples correlated with the amount of methane produced in the breath test, suggesting that M. smithii may be the main methanogen responsible for the methane detected in human breath tests [54].
In the studies conducted by Ghoshal et al., it was found that the number of copies of M. smithii was higher among IBS patients, especially IBS-C, than with IBS-D and the control group [57].
Activation of the hypothalamic–pituitary–adrenal (HPA) axis by stress causes the release of CRH from the paraventricular nucleus of the hypothalamus, followed by the release of ACTH from the anterior pituitary. ACTH stimulates the adrenal cortex to release cortisol, which inhibits the vagal response.
The vagus nerve is typically stimulated in its anatomical region of the ear, i.e., in the cymba concha and cavity of concha. The most popular non-invasive forms of vagus nerve stimulation are percutaneous methods by applying stimulating electrodes on the ear skin or minimally invasive methods such as acupuncture.
Stimulation aims to transmit an impulse to the area of NTS and DMV sensory-motor nuclei of the dorsal vagal complex and transfer information from the NTS to the DMV. Stimulation of the vagus nerve in the Concha area alters the vagus nerve’s functional output and activation of the parasympathetic system.
The vagus nerve recognizes the intestinal microflora and transmits this information to the central nervous system. Stimulation of the vagus nerve through non-invasive or minimally invasive methods (acupuncture) leads to restoring the balance of the intestinal microflora. Additionally, the balance of the intestinal microflora is restored through oral intake of nutraceuticals. Created with Biorender.com accessed on 1 September 2023.
3. The Role of Serotonin in IBS
Numerous studies confirm that serotonin plays a key role in intestinal neurotransmission, initiation and propagation of gastrointestinal motor activity, and gut–brain signaling [8].
Other studies report that the hormone serotonin 5-hydroxytryptamine (5-HT) may also modulate visceral perception. Serotonin in the intestine is released from cells (EC). It is believed that the 5-HT3 and 5-HT4 receptors may play an essential role in transmitting visceral sensation from the gut [58]. It is well known that 95% of the organism’s serotonin is produced in the gut, where it has been increasingly examined for its paracrine, autocrine, and hormonal effects [59].
The work of Bulbring et al. highlighted for the first time the link between 5-HT, the peristaltic movement, and the stimulation of peristaltic waves. In isolated preparations of the guinea pig ileum, it was shown that synthetic intestinal 5-HT instigates the peristaltic motion and, further, that stimulation of the peristaltic movement causes 5-HT to be secreted in the gut [59]. In connection with the above, there must be a strong relationship between IBS pathogenesis (5-HT) and the serotonin transporter (SERT) [60]. As a sodium neurotransmitter and symporter family member, SERT regulates the extracellular availability of 5-HT in the intestine through 5-HT uptake. Genetic or environmental abnormality of SERT expression is associated with abnormal 5-HT levels in the intestinal mucosa and may contribute to the development of functional gastrointestinal diseases such as IBS [61]. 5-hydroxytryptamine (5-HT) is a messenger for intestinal motility, visceral sensation, and fluid secretion. SERT is also a transmembrane transporter with a high affinity for serotonin and plays a vital role in its metabolism. High serotonin levels are often associated with reduced SERT levels in IBS patients. Several studies found that SERT polymorphisms were closely associated with IBS risk in Caucasians and Asians whose serotonin levels in rectal mucosa biopsies were high, particularly in IBS-D [60].
Interestingly, an excess of serotonin in the gut may result from a protective response to infection, facilitating the rapid clearance of infecting organisms from the gut. One prospective study showed that people who developed PI-IBS after infection with C. jejuni had a significant increase in both mucosal CD3-positive lymphocytes and EC cells compared to controls [1].
Animal studies have shown that SERT knockout mice had diarrhea associated with colon hypermotility, resulting in increased water excretion in the feces [62]. Wang et al. indicated that SERT polymorphisms may contribute to the development of IBS [63]. The research so far focuses on genetic polymorphisms related to the etiology of IBS, such as SLC6A4, 5-HTTLPR, or a variable number of tandem repeats STin2 [64].
4. Plant Metabolites in IBS Treatment
Plant metabolites are used to reduce the severity of IBS symptoms (Figure 2). Several scientific publications describe the excellent performance of soya isoflavones and vitamin D, which allow for a reduction in plasma inflammatory markers and fecal protease activity in women with IBS [65,66]. Women between 18 and 75 years old with IBS were included in the study. Participants were randomly divided into four groups of 25 women each (placebo of vitamin D and placebo of soy isoflavones, placebo of vitamin D and soy isoflavones, vitamin D and placebo of soy isoflavones, and vitamin D and soy isoflavones) and the therapy was administered for 6 weeks. A validated IBS symptoms severity score (IBS-SSS) questionnaire and a validated IBS quality of life (IBS-QOL) questionnaire were used to verify the results. The authors reported that the effect of isoflavones persisted even after supplementation against abdominal pain and flatulence was discontinued. The soy phytoestrogens and vitamin D bind to ERs in smooth muscle cells and modulate the expression of ER proteins. Vitamin D ensures homeostasis of the intestinal mucosal barrier, and vitamin D deficiency is related to severe IBS. A significant reduction in TNF-α in the “soy with vitamin D” and “soy” groups was also found compared to the placebo group. The leukocyte nuclear factor-κβ level NF-κβ levels and fecal protease activity were significantly lower in all three treatment groups.
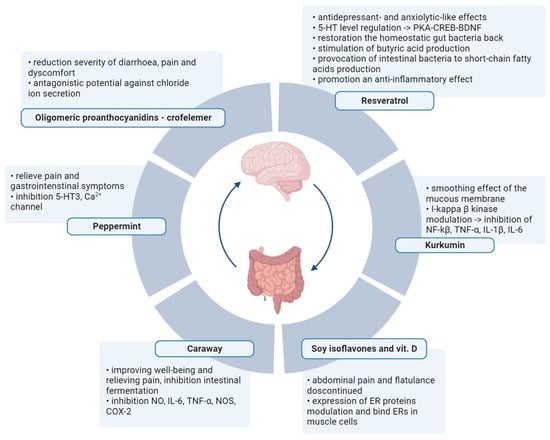
Figure 2. Alternative therapies in IBS with the use of active metabolites of plants. Normalization of the intestinal microflora and elimination of intestinal pathogens through oral intake of nutraceuticals. Created with BioRender.com, accessed on 1 September 2023.
This entry is adapted from the peer-reviewed paper 10.3390/ph16101405
This entry is offline, you can click here to edit this entry!
