As humans expand their territories across more and more regions of the planet, activities such as deforestation, urbanization, tourism, wildlife exploitation, and climate change can have drastic consequences for animal movements and animal–human interactions. These events, especially climate change, can also affect the arthropod vectors that are associated with the animals in these scenarios. As the COVID-19 pandemic and other various significant outbreaks throughout the centuries have demonstrated, when animal patterns and human interactions change, so does the exposure of humans to zoonotic pathogens potentially carried by wildlife.
- zoonoses
- emerging
- climate change
- urbanization
- deforestation
- wildlife exploitation
1. Introduction
2. Urbanization
3. Deforestation
Deforestation modifies wildlife communities, and thus increasing zoonotic spillover potential is a growing area of concern [13]. The increase in zoonotic outbreaks, majorly in tropical lands, has been in part connected to human population growth from 1990 to 2016 [14]. To help reduce the risk of the emergence and spread of zoonotic pathogens, it would be of great value to encourage the redirection of wildlife hosts to alternate forested lands when they have lost habitat space in a deforested region [13]. The regeneration and reparation of forested lands can reduce the transmission potential of zoonoses while also benefiting the ecosystem as a whole [13]. New studies have shown that viruses are more likely to be transmitted from animals to humans if they are around disturbed ecosystems, which include deforested lands [14]. These outbreaks can lead to an increase in zoonotic pandemics as well. In 2013, the Ebola outbreak emerged after an eighteen-month-old child became ill while playing near a tree and died shortly thereafter [15]. The World Health Organization reported that the Ebola outbreak may have been caused by deforestation, which caused bats to infest the child’s village [16]. Large portions of the bats’ biological environment were destroyed due to deforestation, forcing them to infiltrate the village [17]. Another example is the coronavirus, SARS-CoV-2, which originated in an animal, most likely a bat, and then spread globally causing a pandemic that killed millions of infected patients [18][19]. The rising increase in deforestation causes ecological landscape disruptions, loss of biodiversity, and displaced wildlife migrations to local communities, all of which are associated with increases in zoonotic transmissions [20].
4. Tourism and Zoos
5. Wildlife Exploitation and Trade
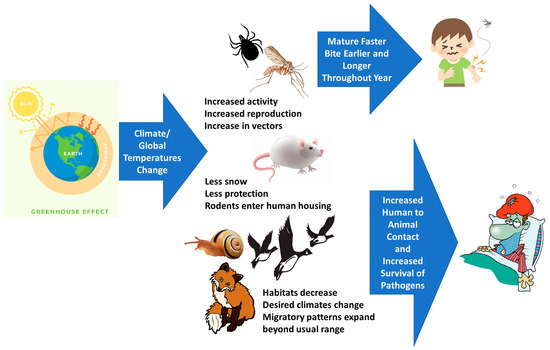
6. Climate Change
This entry is adapted from the peer-reviewed paper 10.3390/ani13101646
References
- White, R.J.; Razgour, O. Emerging zoonotic diseases originating in mammals: A systematic review of effects of anthropogenic land-use change. Mammal Rev. 2020, 50, 336–352.
- Cavallero, S.; Gabrielli, S.; Gazzonis, A.L.; Pombi, M.; Šnábel, V. Editorial: Zoonotic Parasitic Diseases in a Changing World. Front. Vet. Sci. 2021, 8, 715112.
- Figueroa, D.; Duprat, X. Remedying anthropogenic zoonoses. Anim. Sentience 2020, 5, 29.
- Esposito, A.M.; Esposito, M.M.; Ptashnik, A. Phylogenetic Diversity of Animal Oral and Gastrointestinal Viromes Useful in Surveillance of Zoonoses. Microorganisms 2022, 10, 1815.
- Ostfeld, R.S. Biodiversity loss and the rise of zoonotic pathogens. Clin. Microbiol. Infect. 2009, 15, 40–43.
- Keesing, F.; Ostfeld, R.S. Impacts of biodiversity and biodiversity loss on zoonotic diseases. Proc. Natl. Acad. Sci. USA 2021, 118, e2023540118.
- Mackenstedt, U.; Jenkins, D.; Romig, T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites Wildl. 2015, 4, 71–79.
- Wood, C.L.; McInturff, A.; Young, H.S.; Kim, D.; Lafferty, K.D. Human infectious disease burdens decrease with urbanization but not with biodiversity. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160122.
- Crompton, D.W.; Savioli, L. Intestinal parasitic infections and urbanization. Bull. World Health Organ. 1993, 71, 1.
- Fernando, S.U.; Udagama, P.; Fernando, S.P. Effect of urbanization on zoonotic gastrointestinal parasite prevalence in endemic toque macaque (Macaca sinica) from different climatic zones in Sri Lanka. Int. J. Parasitol. Parasites Wildl. 2022, 17, 100–109.
- Patwary, M.M.; Haque, M.d.Z.; Bardhan, M.; Rodriguez-Morales, A.J. COVID-19 and Dengue Co-epidemic During the Second Wave of the Pandemic in Bangladesh: A Double Blow for an Overburdened Health-Care System. Disaster Med. Public Health Prep. 2022, 16, 2235–2237.
- Plumer, L.; Davison, J.; Saarma, U. Rapid Urbanization of Red Foxes in Estonia: Distribution, Behaviour, Attacks on Domestic Animals, and Health-Risks Related to Zoonotic Diseases. PLoS ONE 2014, 9, e115124.
- Vinson, J.E.; Gottdenker, N.L.; Chaves, L.F.; Kaul, R.B.; Kramer, A.M.; Drake, J.M.; Hall, R.J. Land reversion and zoonotic spillover risk. R. Soc. Open Sci. 2022, 9, 220582.
- Morand, S.; Lajaunie, C. Outbreaks of Vector-Borne and Zoonotic Diseases Are Associated With Changes in Forest Cover and Oil Palm Expansion at Global Scale. Front. Vet. Sci. 2021, 8, 230.
- Marí Saéz, A.; Weiss, S.; Nowak, K.; Lapeyre, V.; Zimmermann, F.; Düx, A.; Kühl, H.S.; Kaba, M.; Regnaut, S.; Merkel, K.; et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol. Med. 2015, 7, 17–23.
- Baudel, H.; De Nys, H.; Mpoudi Ngole, E.; Peeters, M.; Desclaux, A. Understanding Ebola virus and other zoonotic transmission risks through human–bat contacts: Exploratory study on knowledge, attitudes and practices in Southern Cameroon. Zoonoses Public Health 2019, 66, 288–295.
- Olivero, J.; Fa, J.E.; Farfán, M.Á.; Márquez, A.L.; Real, R.; Juste, F.J.; Leendertz, S.A.; Nasi, R. Human activities link fruit bat presence to Ebola virus disease outbreaks. Mammal Rev. 2020, 50, 1–10.
- Zhou, H.; Ji, J.; Chen, X.; Bi, Y.; Li, J.; Wang, Q.; Hu, T.; Song, H.; Zhao, R.; Chen, Y.; et al. Identification of novel bat coronaviruses sheds light on the evolutionary origins of SARS-CoV-2 and related viruses. Cell 2021, 184, 4380–4391.e14.
- Zhou, H.; Chen, X.; Hu, T.; Li, J.; Song, H.; Liu, Y.; Wang, P.; Liu, D.; Yang, J.; Holmes, E.C.; et al. A Novel Bat Coronavirus Closely Related to SARS-CoV-2 Contains Natural Insertions at the S1/S2 Cleavage Site of the Spike Protein. Curr. Biol. 2020, 30, 2196–2203.e3.
- Tajudeen, Y.A.; Oladunjoye, I.O.; Bajinka, O.; Oladipo, H.J. Zoonotic Spillover in an Era of Rapid Deforestation of Tropical Areas and Unprecedented Wildlife Trafficking: Into the Wild. Challenges 2022, 13, 41.
- Mavroidi, N. Transmission of zoonoses through immigration and tourism. Vet. Ital. 2008, 44, 651–656.
- Green, J.; Jakins, C.; Asfaw, E.; Bruschi, N.; Parker, A.; de Waal, L.; D’Cruze, N. African Lions and Zoonotic Diseases: Implications for Commercial Lion Farms in South Africa. Animals 2020, 10, 1692.
- Caballero-Gómez, J.; Cano-Terriza, D.; Lecollinet, S.; Carbonell, M.D.; Martínez-Valverde, R.; Martínez-Nevado, E.; García-Párraga, D.; Lowenski, S.; García-Bocanegra, I. Evidence of exposure to zoonotic flaviviruses in zoo mammals in Spain and their potential role as sentinel species. Vet. Microbiol. 2020, 247, 108763.
- Stirling, J.; Griffith, M.; Dooley, J.S.G.; Goldsmith, C.E.; Loughrey, A.; Lowery, C.J.; McClurg, R.; McCorry, K.; McDowell, D.; McMahon, A.; et al. Zoonoses Associated with Petting Farms and Open Zoos. Vector-Borne Zoonotic Dis. 2008, 8, 85–92.
- Cuenca, P.R.; Key, S.; Jumail, A.; Surendra, H.; Ferguson, H.M.; Drakeley, C.J.; Fornace, K. Chapter Six—Epidemiology of the zoonotic malaria Plasmodium knowlesi in changing landscapes. In Advances in Parasitology; Drakeley, C., Ed.; Current Research on Naturally Transmitted; Academic Press: Cambridge, MA, USA, 2021; Volume 113, pp. 225–286.
- Norris, D.E. Mosquito-borne Diseases as a Consequence of Land Use Change. EcoHealth 2004, 1, 19–24.
- Molyneaux, A.; Hankinson, E.; Kaban, M.; Svensson, M.S.; Cheyne, S.M.; Nijman, V. Primate Selfies and Anthropozoonotic Diseases: Lack of Rule Compliance and Poor Risk Perception Threatens Orangutans. Folia Primatol. 2021, 92, 296–305.
- Lenzi, C.; Speiran, S.; Grasso, C. “Let Me Take a Selfie”: Implications of Social Media for Public Perceptions of Wild Animals. Soc. Anim. 2020, 31, 64–83.
- D’Cruze, N.; Green, J.; Elwin, A.; Schmidt-Burbach, J. Trading Tactics: Time to Rethink the Global Trade in Wildlife. Animals 2020, 10, 2456.
- Aguirre, A.A.; Catherina, R.; Frye, H.; Shelley, L. Illicit Wildlife Trade, Wet Markets, and COVID-19: Preventing Future Pandemics. World Med. Health Policy 2020, 12, 256–265.
- Morcatty, T.Q.; Pereyra, P.E.R.; Ardiansyah, A.; Imron, M.A.; Hedger, K.; Campera, M.; Nekaris, K.A.-I.; Nijman, V. Risk of Viral Infectious Diseases from Live Bats, Primates, Rodents and Carnivores for Sale in Indonesian Wildlife Markets. Viruses 2022, 14, 2756.
- Pavlin, B.I.; Schloegel, L.M.; Daszak, P. Risk of Importing Zoonotic Diseases through Wildlife Trade, United States. Emerg. Infect. Dis. 2009, 15, 1721–1726.
- Hooper, J. Contamination: The Case of Civets, Companionship, COVID, and SARS. J. Appl. Anim. Welf. Sci. 2022, 25, 167–179.
- Rush, E.R.; Dale, E.; Aguirre, A.A. Illegal Wildlife Trade and Emerging Infectious Diseases: Pervasive Impacts to Species, Ecosystems and Human Health. Animals 2021, 11, 1821.
- Rossi, G.; Aubry, P.; Dubé, C.; Smith, R.L. The spread of bovine tuberculosis in Canadian shared pastures: Data, model, and simulations. Transbound. Emerg. Dis. 2019, 66, 562–577.
- Moyen, N.; Hoque, M.A.; Mahmud, R.; Hasan, M.; Sarkar, S.; Biswas, P.K.; Mehedi, H.; Henning, J.; Mangtani, P.; Flora, M.S.; et al. Avian influenza transmission risk along live poultry trading networks in Bangladesh. Sci. Rep. 2021, 11, 19962.
- Koh, L.P.; Li, Y.; Lee, J.S.H. The value of China’s ban on wildlife trade and consumption. Nat. Sustain. 2021, 4, 2–4.
- Directive No. 29/CT-TTg on a Number of Urgent Solutions for Wildlife Management. Available online: https://english.luatvietnam.vn/directive-no-29-ct-ttg-on-a-number-of-urgent-solutions-for-wildlife-management-187252-doc1.html (accessed on 1 April 2023).
- Halbwax, M. Addressing the illegal wildlife trade in the European Union as a public health issue to draw decision makers attention. Biol. Conserv. 2020, 251, 108798.
- van Vliet, N.; Muhindo, J.; Nyumu, J.; Enns, C.; Massé, F.; Bersaglio, B.; Cerutti, P.; Nasi, R. Understanding Factors that Shape Exposure to Zoonotic and Food-Borne Diseases Across Wild Meat Trade Chains. Hum. Ecol. 2022, 50, 983–995.
- Ben Chehida, F.; Gharsa, H.; Tombari, W.; Selmi, R.; Khaldi, S.; Daaloul, M.; Ben Slama, K.; Messadi, L. First Report of Antimicrobial Susceptibility and Virulence Gene Characterization Associated with Staphylococcus aureus Carriage in Healthy Camels from Tunisia. Animals 2021, 11, 2754.
- Gong, Q.-L.; Chen, Y.; Tian, T.; Wen, X.; Li, D.; Song, Y.-H.; Wang, Q.; Du, R.; Zhang, X.-X. Prevalence of bovine tuberculosis in dairy cattle in China during 2010–2019: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009502.
- Moise-Silverman, J. Zoonotic Disease Surveillance and Response: Is There a Duty to Intervene when a Disease is Detected? Int. J. Infect. Dis. 2022, 116, S77.
- Kim, S.-H. Challenge for One Health: Co-Circulation of Zoonotic H5N1 and H9N2 Avian Influenza Viruses in Egypt. Viruses 2018, 10, 121.
- Sachan, N.; Singh, V.P. Effect of climatic changes on the prevalence of zoonotic diseases. Vet. World 2010, 3, 519.
- Naicker, P.R. The impact of climate change and other factors on zoonotic diseases. Arch. Clin. Microbiol. 2011, 2, 4.
- Tajudeen, Y.A.; Oladunjoye, I.O.; Adebayo, A.O.; Adebisi, Y.A. The need to adopt planetary health approach in understanding the potential influence of climate change and biodiversity loss on zoonotic diseases outbreaks. Public Health Pract. 2021, 2, 100095.
- Singh, B.; Sharma, R.; Gill, J.; Aulakh, R.S.; Banga, H.S. Climate change, zoonoses and India. Rev. Sci. Tech. Int. Off. Epizoot. 2011, 30, 779–788.
- Lindgren, E.; Gustafson, R. Tick-borne encephalitis in Sweden and climate change. Lancet 2001, 358, 16–18.
- Parkinson, A.J.; Evengard, B.; Semenza, J.C.; Ogden, N.; Børresen, M.L.; Berner, J.; Brubaker, M.; Sjöstedt, A.; Evander, M.; Hondula, D.M.; et al. Climate change and infectious diseases in the Arctic: Establishment of a circumpolar working group. Int. J. Circumpolar Health 2014, 73, 25163.
- Hueffer, K.; Parkinson, A.J.; Gerlach, R.; Berner, J. Zoonotic infections in Alaska: Disease prevalence, potential impact of climate change and recommended actions for earlier disease detection, research, prevention and control. Int. J. Circumpolar Health 2013, 72, 19562.
- Khan, M.D.; Thi Vu, H.H.; Lai, Q.T.; Ahn, J.W. Aggravation of Human Diseases and Climate Change Nexus. Int. J. Environ. Res. Public. Health 2019, 16, 2799.
- Tian, H.; Zhou, S.; Dong, L.; Van Boeckel, T.P.; Pei, Y.; Wu, Q.; Yuan, W.; Guo, Y.; Huang, S.; Chen, W.; et al. Climate change suggests a shift of H5N1 risk in migratory birds. Ecol. Model. 2015, 306, 6–15.
- Park, B.J.; Sigel, K.; Vaz, V.; Komatsu, K.; McRill, C.; Phelan, M.; Colman, T.; Comrie, A.C.; Warnock, D.W.; Galgiani, J.N.; et al. An Epidemic of Coccidioidomycosis in Arizona Associated with Climatic Changes, 1998–2001. J. Infect. Dis. 2005, 191, 1981–1987.
- Estrada-Peña, A.; Ostfeld, R.S.; Peterson, A.T.; Poulin, R.; de la Fuente, J. Effects of environmental change on zoonotic disease risk: An ecological primer. Trends Parasitol. 2014, 30, 205–214.
- Rupasinghe, R.; Chomel, B.B.; Martínez-López, B. Climate change and zoonoses: A review of the current status, knowledge gaps, and future trends. Acta Trop. 2022, 226, 106225.
- Hellberg, R.S.; Chu, E. Effects of climate change on the persistence and dispersal of foodborne bacterial pathogens in the outdoor environment: A review. Crit. Rev. Microbiol. 2016, 42, 548–572.
- Anyamba, A.; Chretien, J.-P.; Britch, S.C.; Soebiyanto, R.P.; Small, J.L.; Jepsen, R.; Forshey, B.M.; Sanchez, J.L.; Smith, R.D.; Harris, R.; et al. Global Disease Outbreaks Associated with the 2015–2016 El Niño Event. Sci. Rep. 2019, 9, 1930.
- Sipari, S.; Khalil, H.; Magnusson, M.; Evander, M.; Hörnfeldt, B.; Ecke, F. Climate change accelerates winter transmission of a zoonotic pathogen. Ambio 2022, 51, 508–517.
- Nosrat, C.; Altamirano, J.; Anyamba, A.; Caldwell, J.M.; Damoah, R.; Mutuku, F.; Ndenga, B.; LaBeaud, A.D. Impact of recent climate extremes on mosquito-borne disease transmission in Kenya. PLoS Negl. Trop. Dis. 2021, 15, e0009182.
- Huber, I.; Potapova, K.; Ammosova, E.; Beyer, W.; Blagodatskiy, S.; Desyatkin, R.; Hoelzle, L.E.; Ignateva, M.; Kokolova, L.; Lemke, S.; et al. Symposium report: Emerging threats for human health—Impact of socioeconomic and climate change on zoonotic diseases in the Republic of Sakha (Yakutia), Russia. Int. J. Circumpolar Health 2020, 79, 1715698.
- Zang, S.M.; Benjenk, I.; Breakey, S.; Pusey-Reid, E.; Nicholas, P.K. The intersection of climate change with the era of COVID-19. Public Health Nurs. 2021, 38, 321–335.
- Marazziti, D.; Cianconi, P.; Mucci, F.; Foresi, L.; Chiarantini, I.; Della Vecchia, A. Climate change, environment pollution, COVID-19 pandemic and mental health. Sci. Total Environ. 2021, 773, 145182.
- Mishra, J.; Mishra, P.; Arora, N.K. Linkages between environmental issues and zoonotic diseases: With reference to COVID-19 pandemic. Environ. Sustain. 2021, 4, 455–467.
