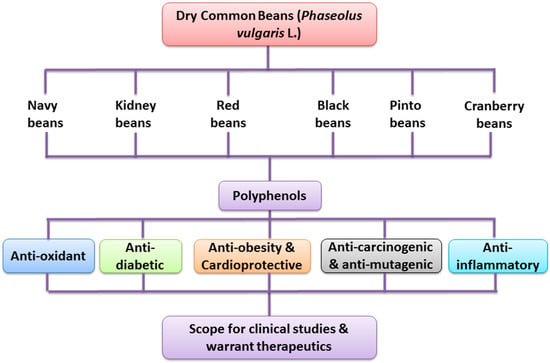
Polyphenols are plant metabolites with potent anti-oxidant properties, which help to reduce the effects of oxidative stress-induced dreaded diseases. The evidence demonstrated that dietary polyphenols are of emerging increasing scientific interest due to their role in the prevention of degenerative diseases in humans. Possible health beneficial effects of polyphenols are based on the human consumption and their bioavailability. Common beans (Phaseolus vulgaris L.) are a greater source of polyphenolic compounds with numerous health promoting properties. Polyphenol-rich dry common beans have potential effects on human health, and possess anti-oxidant, anti-diabetic, anti-obesity, anti-inflammatory and anti-mutagenic and anti-carcinogenic properties.
- polyphenols
- Phaseolus vulgaris
- anti-oxidants
- degenerative diseases
- health-promoting effects
1. Introduction
2. Common Beans and Their Health Benefits
3. Nutritional Compositions of Common Beans
|
Nutrient |
Units |
Navy Beans |
Kidney Beans |
Red Beans |
Black Beans |
Pinto Beans |
Cranberry Beans |
|---|---|---|---|---|---|---|---|
|
Energy |
kcal |
92 |
92 |
167 |
464 |
500 |
257 |
|
Protein |
g |
6.15 |
5.38 |
22.22 |
14.29 |
10.71 |
22.86 |
|
Total lipid (fat) |
g |
0.00 |
0.38 |
0.00 |
21.43 |
21.43 |
0.00 |
|
Carbohydrate |
g |
16.15 |
20.77 |
63.89 |
57.14 |
60.71 |
60.00 |
|
Total dietary fiber |
g |
4.6 |
5.4 |
44.4 |
14.3 |
10.7 |
25.7 |
|
Total sugars |
g |
0.00 |
0.77 |
2.78 |
3.57 |
3.57 |
2.86 |
|
Resistant starch |
g |
4.2 |
2.0 |
3.8 |
1.7 |
1.9 |
2.6 |
|
Minerals |
|||||||
|
Calcium |
mg |
62 |
46 |
167 |
143 |
71 |
114 |
|
Iron |
mg |
1.38 |
1.38 |
7.29 |
3.86 |
2.57 |
5.1 |
|
Potassium |
mg |
300 |
268 |
222 |
279 |
96 |
265 |
|
Magnesium |
mg |
48 |
37 |
44 |
60 |
43 |
39 |
|
Sodium |
mg |
108 |
8 |
69 |
286 |
286 |
10 |
|
Vitamins |
|||||||
|
Vitamin C |
mg |
0.9 |
0.9 |
0.0 |
0.0 |
0.0 |
0.0 |
|
Folate |
µg |
127 |
115 |
140 |
128 |
147 |
124 |
|
Lipids |
|||||||
|
Total saturated |
g |
0.000 |
0.000 |
0.000 |
1.790 |
1.790 |
0.000 |
|
Total monounsaturated fatty acids |
g |
0.000 |
0.000 |
0.000 |
14.290 |
14.290 |
0.000 |
|
Total polyunsaturated fatty acids |
g |
0.000 |
0.000 |
0.000 |
5.360 |
5.360 |
0.000 |
|
Polyphenol |
mg of gallic acid equiv/g |
12.47 |
14.14 |
13.68 |
12.60 |
12.52 |
11.73 |
|
Flavonoids |
mg of rutin equiv/g |
1.78 |
2.59 |
1.55 |
1.28 |
0.98 |
1.65 |
4. Polyphenols in Common Beans
|
Bean Name |
Polyphenol Class |
Polyphenol Sub-Class |
Compound Name |
References |
|---|---|---|---|---|
|
Dark bean |
Flavonoids |
Anthocyanins |
Cyanidin 3-O-glucoside, pelargonidin 3-O-glucoside, petunidin-3-O-β-glucopyranoside, malvidin 3-O-glucoside, delphinidin acetyl-glucoside, pelargonidin acetyl glucoside, pelargonidin 3-O-malonyl glucoside, petunidin feruloyl glucose |
[40] |
|
Wild and weedy Mexican bean, pinto and black beans |
Flavonoids |
Anthocyanins |
Peonidin, pelargonidin, cyanidin |
|
|
Dark bean, Wild, and weedy Mexican bean |
Flavonoids |
Anthocyanins |
Delphinidin 3-O-glucoside |
|
|
Alubia, black, cranberry, dark red kidney, great northern, light red kidney, navy, pink, pinto, and small red |
Flavonoids |
Anthocyanins |
Petunidin 3-O-(6″-acetyl-glucoside) |
|
|
Dark and kidney bean, zolfino landraces |
Flavonoids |
Anthocyanins |
Pelargonidin 3,5-O-diglucoside |
|
|
Alubia, black, cranberry, dark red kidney, great northern, light red kidney, navy, pink, pinto, and small red |
Flavonoids |
Anthocyanins |
Delphinidin 3-O-glucosyl-glucoside |
|
|
Dark bean |
Flavonoids |
Flavanols |
(+)-Catechin, (-)-epicatechin, (+)-gallocatechin, procyanidin dimer, (-)-epigallocatechin, Procyanidin dimer B2, procyanidin dimer B3, procyanidin dimer B4, procyanidin trimer, procyanidin trimer EEC, naringenin 7-glucoside |
[40] |
|
Dark bean |
Flavonoids |
Flavanones |
Naringenin, hesperetin, naringin, naringenin 7-O-rutinoside, naringenin 7-O-glucoside, naringenin-7-methyl ether 2, hesperetin 3′-O-glucuronide, hesperetin 7-O-glucuronide, hesperetin 3′,7-O-diglucuronide, hesperetin 5,7-O-diglucuronide, hesperetin 7-O-rutinoside |
[40] |
|
Dark bean |
Flavonoids |
Flavones |
Apigenin, apigenin 7-O-glucoside |
[40] |
|
Brazilian bean |
Flavonoids |
Flavones |
Chrysin |
[45] |
|
Dark bean, Brazilian bean, Mexican bean |
Flavonoids |
Flavonols |
Kaempferol |
|
|
Dark bean, Brazilian bean, Mexican bean |
Flavonoids |
Flavonols |
Quercetin |
|
|
Dark bean, and Brazilian bean |
Flavonoids |
Flavonols |
Quercetin 3-O-galactoside, Quercetin 3-O-glucoside, Quercetin 3-O-rutinoside, Myricetin, Myricetin 3-O-glucoside, Myricetin 3-O-rhamnoside, Kaempferol 3-O-glucoside, Kaempferol 3-O-rutinoside |
|
|
Pinto beans, zolfino landraces |
Flavonoids |
Flavonols |
Kaempferol 3-O-glucosylxylose |
|
|
Alubia, black, cranberry, dark red kidney, great northern, light red kidney, navy, pink, pinto, and small red |
Flavonoids |
Flavonols |
Kaempferol 3-O-xylosyl-glucoside |
|
|
Pinto beans |
Flavonoids |
Flavonols |
Kaempferol 3-O-acetyl-glucoside |
[42] |
|
Dark bean, Brazilian bean |
Flavonoids |
Isoflavonoids |
Daidzein |
|
|
Dark bean, Brazilian bean |
Flavonoids |
Isoflavonoids |
Genistein |
|
|
Dark bean |
Flavonoids |
Isoflavonoids |
Biochanin A |
[40] |
|
Pinto and black beans |
Flavonoids |
Isoflavonoids |
Glycitein |
[57] |
|
Dark bean |
Flavonoids |
Isoflavonoids |
Dihydrogenistein |
[40] |
|
Brazilian bean |
Polyphenols |
Polyphenols |
Coumestrol |
[45] |
|
Dark bean, pinto and black beans, Mexican bean |
Phenolic acids |
Hydroxybenzoic acids |
Protocatechuic acid |
|
|
Dark bean |
Phenolic acids |
Hydroxybenzoic acids |
Gallic acid |
[40] |
|
Mexican bean |
Phenolic acids |
Hydroxybenzoic acids |
Vanillic acid |
[33] |
|
Dark bean, Mexican bean |
Phenolic acids |
Hydroxycinnamic acids |
p-Coumaric acid |
|
|
Pinto and black beans |
Phenolic acids |
Hydroxycinnamic acids |
Caffeic acid |
[57] |
|
Dark bean, Mexican bean |
Phenolic acids |
Hydroxycinnamic acids |
Ferulic acid |
|
|
Dark bean |
Phenolic acids |
Hydroxycinnamic acids |
Sinapic acid, Ferulic acid 4-glucoside |
[40] |
|
Dark bean |
Stilbenes |
Stilbenes |
trans-Resveratrol, resveratrol 3-O-glucoside |
[40] |
5. Health Promoting Effects of Polyphenol-Rich Dry Beans
5.1. Anti-Oxidant Activity
5.2. Anti-Diabetic Activity
5.3. Anti-Obesity and Cardioprotective Activity
5.4. Anti-Mutagenic and Anti-Carcinogenic Activities
5.5. Anti-Inflammatory Activity
This entry is adapted from the peer-reviewed paper 10.3390/ijms18112331
References
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in food: Cancer prevention and apoptosis induction. Curr. Med. Chem. 2017.
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9.
- McDougall, G.J. Phenolic-enriched foods: Sources and processing for enhanced health benefits. Proc. Nutr. Soc. 2017, 76, 163–171.
- Aparicio-Fernández, X.; García-Gasca, T.; Yousef, G.G.; Lila, M.A.; González de Mejía, E.; Loarca-Piña, G. Chemopreventive activity of polyphenolics from black Jamapa Bean (Phaseolus vulgaris L.) on HeLa and HaCaT cells. J. Agric. Food Chem. 2006, 54, 2116–2122.
- Aparicio-Fernández, X.; Yousef, G.G.; Loarca-Piña, G.; González de Mejía, E.; Lila, M.A. Characterization of polyphenolics in the seed coat of Black Jamapa bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2005, 53, 4615–4622.
- Aparicio-Fernández, X.; Manzo-Bonilla, L.; Loarca-Piña, G. Comparison of antimutagenic activity of phenolic compounds in newly harvested and stored common beans Phaseolus Vulgaris against aflatoxin B1. J. Food Sci. 2005, 70, S73–S78.
- Beninger, C.W.; Hosfield, G.L. Antioxidant activity of extracts, condensed tannin fractions, and pure flavonoids from Phaseolus vulgaris L. seed coat color genotypes. J. Agric. Food Chem. 2003, 51, 7879–7883.
- Cardador-Martínez, A.; Loarca-Piña, G.; Oomah, B.D. Antioxidant activity in common beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2002, 50, 6975–6980.
- Guzmán-Maldonado, S.H.; Paredes-López, O. Functional products of plants indigenous of Latin America: Amaranth, quinoa, common beans and botanicals. In Functional Foods. Bichemical and Processing Aspects; Mazza, G., Ed.; Thechnomic: Lancaster, PA, USA, 1998; pp. 39–328.
- Hangen, L.; Bennink, M.R. Consumption of Black Beans and Navy Beans (Phaseolus vulgaris) Reduced azoxymethane-induced colon cancer in rats. Nutr. Cancer 2002, 44, 60–65.
- Queiroz-Monici, K.S.; Costa, G.E.A.; da Silva, N.; Reis, S.M.P.M.; de Oliveira, A.C. Bifidogenic effect of dietary fiber and resistant starch from leguminous on the intestinal microbiota of rats. Nutrition 2005, 21, 602–609.
- FAO. Estadísticas de Fríjol Seco. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 26 May 2014).
- Adams, M.W. Energy inputs in dry bean production. In Handbook of Energy Utilization in Agriculture; Pimentel, D., Ed.; CRC Press: Boca Raton, FL, USA, 1980; pp. 123–126.
- Mitchell, D.C.; Lawrence, F.R.; Hartman, T.J.; Curran, J.M. Consumption of dry beans, peas, and lentils could improve diet quality in the US population. J. Am. Diet. Assoc. 2009, 109, 909–913.
- Zhang, C.; Monk, J.M.; Lu, J.T.; Zarepoor, L.; Wu, W.; Liu, R.; Pauls, K.P.; Wood, G.A.; Robinson, L.; Tsao, R.; et al. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br. J. Nutr. 2014, 111, 1549–1563.
- Borresen, E.C.; Brown, D.G.; Harbison, G.; Taylor, L.; Fairbanks, A.; O’Malia, J.; Bazan, M.; Rao, S.; Bailey, S.M.; Wdowik, M.; et al. A randomized controlled trial to increase navy bean or rice bran consumption in colorectal cancer survivors. Nutr. Cancer 2016, 68, 1269–1280.
- Borresen, E.C.; Jenkins-Puccetti, N.; Schmitz, K.; Brown, D.G.; Pollack, A.; Fairbanks, A.; Wdowik, M.; Rao, S.; Nelson, T.L.; Luckasen, G.; et al. A pilot randomized controlled clinical trial to assess tolerance and efficacy of navy bean and rice bran supplementation for lowering cholesterol in children. Glob. Pediatr. Health 2017, 4.
- Lestari, L.A.; Huriyati, E.; Marsono, Y. The development of low glycemic index cookie bars from foxtail millet (Setaria italica), arrowroot (Maranta arundinacea) flour, and kidney beans (Phaseolus vulgaris). J. Food Sci. Technol. 2017, 54, 1406–1413.
- Monk, J.M.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Liu, R.; Pauls, K.P.; Wood, G.A.; Tsao, R.; Robinson, L.E.; et al. White, and dark kidney beans reduce colonic mucosal damage and inflammation in response to dextran sodium sulfate. J. Nutr. Biochem. 2015, 26, 752–760.
- Chao, W.W.; Chung, Y.C.; Shih, I.P.; Wang, H.Y.; Chou, S.T.; Hsu, C.K. Red bean extract inhibits lipopolysaccharide-induced inflammation and H2O2-induced oxidative stress in RAW 264.7 macrophages. J. Med. Food. 2015, 18, 724–730.
- Chan, Y.S.; Ng, T.B. Northeast red beans produce a thermostable and pH-stable defensin-like peptide with potent antifungal activity. Cell Biochem. Biophys. 2013, 66, 637–648.
- Chan, Y.S.; Wong, J.H.; Fang, E.F.; Pan, W.; Ng, T.B. A hemagglutinin from northeast red beans with immunomodulatory activity and anti-proliferative and apoptosis-inducing activities toward tumor cells. Protein Pept. Lett. 2013, 20, 1159–1169.
- Mojica, L.; Berhow, M.; Gonzalez de Mejia, E. Black bean anthocyanin-rich extracts as food colorants: Physicochemical stability and antidiabetes potential. Food Chem. 2017, 229, 628–639.
- Yin, C.; Wong, J.H.; Ng, T.B. Isolation of a hemagglutinin with potent antiproliferative activity and a large antifungal defensin from Phaseolus vulgaris cv. Hokkaido Large Pinto Beans. J. Agric. Food Chem. 2015, 63, 5439–5448.
- Ojeda, A.G.; Wrobel, K.; Escobosa, A.R.; Elguera, J.C.; Garay-Sevilla, M.E.; Wrobel, K. Molybdenum and copper in four varieties of common bean (Phaseolus vulgaris): New data of potential utility in designing healthy diet for diabetic patients. Biol. Trace Elem. Res. 2015, 163, 244–254.
- Monk, J.M.; Lepp, D.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Pauls, K.P.; Tsao, R.; Wood, G.A.; Robinson, L.E.; et al. Diets enriched with cranberry beans alter the microbiota and mitigate colitis severity and associated inflammation. J. Nutr. Biochem. 2016, 28, 129–139.
- Haddad, E.H.; Tanzman, J.S. What do vegetarians in the United States eat? Am. J. Clin. Nutr. 2003, 78, 626–632.
- Messina, V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 2014, 100, 437–442.
- Chávez-Mendoza, C.; Sánchez, E. Bioactive compounds from Mexican varieties of the common bean (Phaseolus vulgaris): Implications for health. Molecules 2017, 22, 1360.
- Suárez-Martínez, S.E.; Ferriz-Martínez, R.A.; Campos-Vega, R.; Elton-Puente, J.E.; de la Torre Carbot, K.; García-Gasca, T. Bean seeds: Leading nutraceutical source for human health. CyTA J. Food 2016, 14, 131–137.
- Ulloa, J.A.; Rosas, U.P.; Ramírez, R.J.C.; Rangel, U.B.E. El frijol (Phaseolus vulgaris): Su importancia nutricionaly como fuente de fitoquímicos. . Rev. Fuente 2011, 3, 5–9.
- Mederos, Y. Indicadores de la calidad en el grano de frijol (Phaseolus vulgaris L.). . Cultiv. Trop. 2006, 27, 55–63.
- Díaz-Batalla, L.; Widholm, J.M.; Fahey, G.C.; Castaño-Tostado, E.; Paredes-López, O. Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 2045–2052.
- Machado, C.M.; Ferruzzi, M.G.; Nielsen, S.S. Impact of the hard-to-cook phenomenon on phenolic antioxidants in dry beans (Phaseolus vulgaris). J. Agric. Food Chem. 2008, 56, 3102–3110.
- Aguilera, Y.; Estrella, I.; Benitez, V.; Esteban, R.M.; Martin-Cabrejas, M.A. Bioactive phenolic compounds and functional properties of dehydrated beans flours. Food Res. Int. 2010, 44, 774–780.
- López-Amorós, M.L.; Hernández, T.; Estrella, I. Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Compos. Anal. 2006, 19, 277–283.
- United States Department of Agriculture (USDA). Agricultural Research Service, National Nutrient Database for Standard Reference Release 28. Nutrient Database Laboratory Homepage. Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 14 July 2016).
- Golam Masum Akond, A.S.M.; Khandaker, L.; Berthold, J.; Gates, J.; Peters, K.; Delong, H.; Hossain, K. Anthocyanin, total polyphenols and antioxidant activity of common bean. Am. J. Food Technol. 2011, 6, 385–394.
- Ren, S.C.; Liu, Z.L.; Wang, P. Proximate composition and flavonoids content and in vitro antioxidant activity of 10 varieties of legume seeds grown in China. J. Med. Plants Res. 2012, 6, 301–308.
- López, A.; El-Naggar, T.; Dueñas, M.; Ortega, T.; Estrella, I.; Hernández, T.; Gómez-Serranillos, M.P.; Palomino, O.M.; Carretero, M.E. Effect of cooking and germination on phenolic composition and biological properties of dark beans (Phaseolus vulgaris L.). Food Chem. 2013, 138, 547–555.
- Cardador-Martinez, A.; Castano-Tostado, E.; Loarca-Pina, G. Antimutagenic activity of natural phenolic compounds present in the common bean (Phaseolus vulgaris) against aflatoxin B1. Food Addit. Contam. 2002, 19, 62–69.
- Beninger, C.W.; Gu, L.; Prior, R.L.; Junk, D.C.; Vandenberg, A.; Bett, K.E. Changes in polyphenols of the seed coat during the after-darkening process in pinto beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2005, 53, 7777–7782.
- Xu, B.J.; Chang, S.K. Total phenolic content and antioxidant properties of Eclipse black beans (Phaseolus vulgaris L.) as affected by processing methods. J. Food Sci. 2008, 73, 19–27.
- Akillioglu, H.G.; Karakaya, S. Changes in total phenols, total flavonoids, and antioxidant activities of common beans and pinto beans after soaking, cooking, and in vitro digestion process. Food Sci. Biotechnol. 2010, 19, 633–639.
- de Lima, P.F.; Colombo, C.A.; Chiorato, A.F.; Yamaguchi, L.F.; Kato, M.J.; Carbonell, S.A. Occurrence of isoflavonoids in Brazilian common bean germplasm (Phaseolus vulgaris L.). J. Agric. Food Chem. 2014, 62, 9699–9704.
- Ranilla, L.G.; Genovese, M.I.; Lajolo, F.M. Polyphenols and antioxidant capacity of seed coat and cotyledon from Brazilian and Peruvian bean cultivars (Phaseolus vulgaris L.). J. Agric. Food Chem. 2007, 55, 90–98.
- Ávalos, G.A.; Pérez-Urria, C.E. Metabolismo secundario de plantas. . Reduca (Biología). Ser. Fisiol. Veg. 2009, 2, 119–145.
- Reynoso Camacho, R.; del Carmen Ríos Ugalde, M.; Torres Pacheco, I.; Acosta Gallegos, J.A.; Palomino Salinas, A.C.; Ramos Gómez, M.; González Jasso, E.; Horacio Guzmán, Y.S.H. Common bean (Phaseolus vulgaris L.) consumption and its effects on colon cancer in Sprague–Dawley rats. Agric. Téc. Méx. 2007, 33, 43–52.
- Juárez-López, B.A.; Aparicio-Fernández, X. Polyphenolics concentration and antiradical capacity of common bean varieties (Phaseolus vulgaris L.) after thermal treatment. In Food Science and Food Biotechnology Essentials: A Contemporary Perspective, 1st ed.; Nevárez-Moorillón, G.V., Ortega-Rivas, E., Eds.; Asociación Mexicana de Ciencia de los Alimentos, A.C : Durango, Mexico, 2012; pp. 25–33.
- Lin, L.Z.; Harnly, J.M.; Pastor-Corrales, M.S.; Luthria, D.L. The polyphenolic profiles of common beans (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410.
- Hayat, I.; Ahmad, A.; Masud, T.; Ahmed, A.; Bashir, S. Nutritional and health perspectives of beans (Phaseolus vulgaris L.): An overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 580–592.
- Ariga, T.; Hamano, M. Radical scavenging action and its mode in procyanidins B-1 and B-3 from adzuki beans to peroxyl radicals. Agric. Biol. Chem. 1990, 54, 2499–2504.
- Tsuda, T.; Ohshima, K.; Kawakishi, S.; Osawa, T. Antioxidative pigments isolated from the seeds of Phaseolus vulgaris L. J. Agric. Food Chem. 1994, 42, 248–251.
- Guzman-Maldonado, G.H.; Castellanos, J.; De Mejıa, E.G. Relationship between theoretical and experimentally detected tannin content of common bean Phaseolus vulgaris L. Food Chem. 1996, 55, 333–335.
- De Mejıa, E.G.; Castano-Tostado, E.; Loarca-Pina, G. Antimutagenic effects of natural phenolic compounds in beans. Mutat. Res. 1999, 441, 1–9.
- Espinosa-Alonso, L.G.; Lygin, A.; Widholm, J.M.; Valverde, M.E.; Paredes-Lopez, O. Polyphenols in wild and weedy Mexican common beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 4436–4444.
- Xu, B.; Chang, S.K. Total phenolic, phenolic acid, anthocyanin, flavan-3-ol, and flavonol profiles and antioxidant properties of pinto and black beans (Phaseolus vulgaris L.) as affected by thermal processing. J. Agric. Food Chem. 2009, 57, 4754–4764.
- Choung, M.G.; Choi, B.R.; An, Y.N.; Chu, Y.H.; Cho, Y.S. Anthocyanin profile of Korean cultivated kidney bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2003, 51, 7040–7043.
- Adebowale, Y.A.; Adeyemi, I.A.; Oshodi, A.A.; Niranjan, K. Isolation, fractionation and characterization of proteins from Mucuna bean. Food Chem. 2007, 104, 287–299.
- Chung, H.J.; Liu, Q.; Pauls, K.P.; Fan, M.Z.; Yada, R. In vitro starch digestibility, expected glycemic index and some physicochemical properties of starch and flour from common bean (Phaseolus vulgaris L.) varieties grown in Canada. Food Res. Int. 2008, 41, 869–875.
- Romani, A.; Vignolini, P.; Galardi, C.; Mulinacci, N.; Benedettelli, S.; Heimler, D. Germplasm characterization of Zolfino landraces (Phaseolus vulgaris L.) by flavonoid content. J. Agric. Food Chem. 2004, 52, 3838–3842.
- Jenkins, A.L. The glycemic index: Looking back 25 years. Cereal Foods World 2007, 52, 50–53.
- Huber, K.; Brigide, P.; Bretas, E.B.; Canniatti-Brazaca, S.G. Phenolic acid, flavonoids and antioxidant activity of common brown beans (Phaseolus vulgaris L.) before and after cooking. J. Nutr. Food Sci. 2016, 6, 1–7.
- Oomah, B.D.; Cardador-Martinez, A.; Loarca-Piña, G. Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L). J. Sci. Food Agric. 2005, 85, 935–942.
- Guajardo-Flores, D.; García-Patiño, M.; Serna-Guerrero, D.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Characterization and quantification of saponins and flavonoids in sprouts, seed coats, and cotyledons of germinated black beans. Food Chem. 2012, 134, 1312–1319.
- Cardador-Martínez, A.; Albores, A.; Bah, M.; Calderón-Salinas, V.; Castaño-Tostado, E.; Guevara-González, R.; Shimada-Miyasaka, A.; Loarca-Piña, G. Relationship among antimutagenic, antioxidant and enzymatic activities of methanolic extract from common beans (Phaseolus vulgaris L). Plant Foods Hum. Nutr. 2006, 61, 161–168.
- Xu, B.; Chang, S.K. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J. Agric. Food Chem. 2008, 56, 7165–7175.
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Materska, M.; Zielińska, E. Antioxidant activity of protein hydrolysates from raw and heat-treated yellow string beans (Phaseolus vulgaris L.). Acta Sci. Pol. Technol. Aliment. 2014, 13, 385–391.
- Frassinetti, S.; Gabriele, M.; Caltavuturo, L.; Longo, V.; Pucci, L. Antimutagenic and antioxidant activity of a selected lectin-free common bean (Phaseolus vulgaris L.) in two cell-based models. Plant Foods Hum. Nutr. 2015, 70, 35–41.
- Venn, B.J.; Mann, J.I. Cereal grains, legumes and diabetes. Eur. J. Clin. Nutr. 2004, 58, 1443–1461.
- Campos-Vega., R.; Loarca-Pina, G.; Oomah, B.D. Minor components of pulses and their potential impact on human health. Food Res. Int. 2010, 43, 461–582.
- Mojica, L.; Meyer, A.; Berhow, M.A.; González de Mejía, E. Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Res. Int. 2015, 69, 38–48.
- Oseguera-Toledo, M.E.; Gonzalez de Mejia, E.; Amaya-Llano, S.L. Hard-to-cook bean (Phaseolus vulgaris L.) proteins hydrolyzed by alcalase and bromelain produced bioactive peptide fractions that inhibit targets of type-2 diabetes and oxidative stress. Food Res. Int. 2015, 76, 839–851.
- Mojica, L.; de Mejía, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016, 7, 713–727.
- Pi-Sunyer, F.X. Pathophysiology and long-term management of the metabolic syndrome. Obes. Res. 2004, 12, 174–180.
- Finley, J.W.; Burrell, J.B.; Reeves, P.G. Pinto bean consumption changes SCFA profiles in fecal fermentations, bacterial populations of the lower bowel, and lipid profiles in blood of humans. J. Nutr. 2007, 137, 2391–2398.
- Anderson, J.W.; Major, A.W. Pulses, and lipaemia, short- and long-term effects: Potential in the prevention of cardiovascular disease. Br. J. Nutr. 2002, 88, 263–271.
- Winham, D.M.; Hutchins, M.H. Baked beans consumption reduces serum cholesterol in hypercholesterolemic adults. Nutr. Res. 2007, 27, 380–386.
- Shutler, S.M.; Bircher, G.M.; Tredger, J.A.; Morgan, L.M.; Walker, A.F.; Low, A.G. The effect of daily baked bean (Phaseolus vulgaris) consumption on the plasma lipid levels of young, normocholesterolemic men. Br. J. Nutr. 1989, 61, 257–265.
- Wu, P.; Liu, S.; Su, J.; Chen, J.; Li, L.; Zhang, R.; Chen, T. Apoptosis triggered by isoquercitrin in bladder cancer cells by activating the AMPK-activated protein kinase pathway. Food Funct. 2017.
- Chatterjee, A.; Ronghe, A.; Padhye, S.B.; Spade, D.A.; Bhat, N.K.; Bhat, H.K. Antioxidant activities of novel resveratrol analogs in breast cancer. J. Biochem. Mol. Toxicol. 2017.
- Thompson, M.D.; Thompson, H.J.; Brick, M.A.; McGinley, J.N.; Jiang, W.; Zhu, Z.; Wolfe, P. Mechanisms associated with dose-dependent inhibition of rat mammary carcinogenesis by dry bean (Phaseolus vulgaris, L.). J. Nutr. 2008, 138, 2091–2097.
- Vergara-Castañeda, H.A.; Guevara-González, R.G.; Ramos-Gómez, M.; Reynoso-Camacho, R.; Guzmán-Maldonado, H.; Feregrino-Pérez, A.A.; Oomah, B.D.; Loarca-Piña, G. Non-digestible fraction of cooked bean (Phaseolus vulgaris L.) cultivar Bayo Madero suppresses colonic aberrant crypt foci in azoxymethane-induced rats. Food Funct. 2010, 1, 294–300.
- Campos-Vega, R.; García-Gasca, T.; Guevara-Gonzalez, R.; Ramos-Gomez, M.; Oomah, B.D.; Loarca-Piña, G. Human gut flora-fermented non-digestible fraction from cooked bean (Phaseolus vulgaris L.) modifies protein expression associated with apoptosis, cell cycle arrest, and proliferation in human adenocarcinoma colon cancer cells. J. Agric. Food Chem. 2012, 60, 12443–12450.
- Correa, P. Epidemiological correlations between diet and cancer frequency. Cancer Res. 1981, 41, 3685–3689.
- Thompson, M.D.; Mensack, M.M.; Jiang, W.; Zhu, Z.; Lewis, M.R.; McGinley, J.N.; Brick, M.A.; Thompson, H.J. Cell signaling pathways associated with a reduction in mammary cancer burden by dietary common bean (Phaseolus vulgaris L.). Carcinogenesis 2012, 33, 226–232.
- Kolonel, L.N.; Hankin, J.H.; Whittemore, A.S.; Wu, A.H.; Gallagher, R.P.; Wilkens, L.R.; John, E.M.; Howe, G.R.; Dreon, D.M.; West, D.W.; et al. Vegetables, fruits, legumes and prostate cancer: A multiethnic case-control study. Cancer Epidemiol. Biomark. Prev. 2000, 9, 795–804.
- Thompson, M.D.; Brick, M.A.; McGinley, J.N.; Thompson, H.J. Chemical composition and mammary cancer inhibitory activity of dry beans. Crop Sci. 2009, 49, 179–186.
- Mensack, M.M.; McGinley, J.N.; Thompson, H.J. Metabolomic analysis of the effects of edible dry beans (Phaseolus vulgaris L.) on tissue lipid metabolism and carcinogenesis in rats. Br. J. Nutr. 2012, 108, 155–165.
- Haydé, V.C.; Ramón, G.G.; Lorenzo, G.O.; Dave, O.B.; Rosalía, R.C.; Paul, W.; Guadalupe, L.P. Non-digestible fraction of beans (Phaseolus vulgaris L.) modulates signaling pathway genes at an early stage of colon cancer in Sprague-Dawley rats. Br. J. Nutr. 2012, 108, 145–154.
- Feregrino-Pérez, A.A.; Berumen, L.C.; García-Alcocer, G.; Guevara-Gonzalez, R.G.; Ramos-Gomez, M.; Reynoso-Camacho, R.; Acosta-Gallegos, J.A.; Loarca-Piña, G. Composition and chemopreventive effect of polysaccharides from common beans (Phaseolus vulgaris L.) on azoxymethane-induced colon cancer. J. Agric. Food Chem. 2008, 56, 8737–8744.
- Feregrino-Perez, A.A.; Piñol-Felis, C.; Gomez-Arbones, X.; Guevara-González, R.G.; Campos-Vega, R.; Acosta-Gallegos, J.; Loarca-Piña, G. A non-digestible fraction of the common bean (Phaseolus vulgaris L.) induces cell cycle arrest and apoptosis during early carcinogenesis. Plant Foods Hum. Nutr. 2014, 69, 248–254.
- Oomah, B.D.; Corbé, A.; Balasubramanian, P. Antioxidant and anti-inflammatory activities of bean (Phaseolus vulgaris L.) hulls. J. Agric. Food Chem. 2010, 58, 8225–8230.
- Gabriele, M.; Pucci, L.; La Marca, M.; Lucchesi, D.; Della Croce, C.M.; Longo, V.; Lubrano, V. A fermented bean flour extract down-regulates LOX-1, CHOP, and ICAM-1 in HMEC-1 stimulated by ox-LDL. Cell Mol. Biol. Lett. 2016, 21, 10–21.
- Reverri, E.J.; Randolph, J.M.; Steinberg, F.M.; Kappagoda, C.T.; Edirisinghe, I.; Burton-Freeman, B.M. Black beans, fiber, and antioxidant capacity pilot study: Examination of whole foods vs. functional components on postprandial metabolic, oxidative stress, and inflammation in adults with metabolic syndrome. Nutrients 2015, 7, 6139–6154.
- Monk, J.M.; Lepp, D.; Wu, W.; Pauls, K.P.; Robinson, L.E.; Power, K.A. Navy and black bean supplementation primes the colonic mucosal microenvironment to improve gut health. J. Nutr. Biochem. 2017, 49, 89–100.
