Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
Driven by its accessibility, extensive availability, and growing environmental consciousness, solid biomass has emerged as a viable alternative to enhance the diversity of renewable energy sources for electricity generation. To understand the phenomena involved in solid biomass conversion, it is necessary not only to understand the stages of the biomass combustion process but also to understand specifically the kinetics of the reaction and the release of the volatiles.
- biomass
- combustion
- kinetics
- macro thermogravimetric analysis
1. Introduction
Energy demand has increased over the years due to population growth and industrial and socio-economic developments, cornerstones of human civilization. As fossil fuels have been the backbone of energy supply worldwide, they are linked with climate change and global warming. Besides the protection of the environment, the depletion of fossil fuel resources is an important aspect that needs to be overcome. Consequently, using alternative energy sources to reduce environmental problems and strain on limited supply is currently a primary concern. As an alternative clean energy source, biomass appears to be a very interesting option as it is considered a sustainable, renewable, and CO2-neutral energy source [1], even though this has been seriously debated recently [2,3,4,5,6]. Furthermore, its abundance and availability not depending on weather conditions make this resource an attractive and valuable alternative for energy supply in both domestic and industrial sectors. Banja et al. [7] noted that biomass is the main contributor to EU renewable energy markets and due to its lower carbon footprint, has a significant contribution to low-carbon economy which results in its key role within the EU policy in the support for renewable energy sources. Furthermore, the International Energy Agency Roadmap—Net Zero Emissions by 2050—identifies bioenergy as an important source of energy, projected to represent 18% of the total energy supply in 2050,playing a key role in the transition toward a carbon-neutral society [8]. This number considers the direct replacement of fossil fuels and, indirectly, the counterbalance emissions by coupling the use of bioenergy with carbon capture and storage. In 2019, the replacement of fossil fuels with biomass avoided 290 MtCO2eq emissions, equivalent to approximately 8% of the EU27 GHG emissions [9].
Nowadays, biomass thermochemical conversion can be categorized into several main types, including combustion, pyrolysis, gasification, and hydrothermal processes. These processes have gained significant interest in recent years due to their potential to produce renewable energy and/or bio-based products while reducing greenhouse gas emissions. Each one has different characteristics and results in different products [10], combustion being the oldest and most mature technology for the production of heat and power. Additionally, the main route for providing renewable heat is solid biomass combustion, typically in grate-fired boilers. The applications of biomass combustion cover a wide range from domestic equipment with dozens of kW to district heating, dedicated, or combined heat and power plants with up to hundreds of MW of installed capacity. The successive climate targets for 2020, 2030, and 2050 have progressively reduced gas emissions targets up to 80% by increasing the share of renewable energy in the energy mix [11]. Nonetheless, there are countries like Portugal, with regard to the biomass sector, that only promote the development of new small co-generation biomass plants (up to 15 MW [12]). The low efficiency of dedicated large-scale biomass plants certainly discourages support for them.
The low efficiency is related to the complexity of the biomass combustion process, its instability, and the non-utilization of the available thermal energy for other purposes. Biomass combustion involves simultaneous multiphase fluid flow, chemical reactions, heat (convection and radiation), and mass transfer [13]. Due to this complex and irregular process, there are various operational problems inside an industrial boiler. Additionally, the pollutant emission limit is often exceeded.
In this regard, in order to give scientific insight into this phenomenon, experiments and Computational Fluid Dynamics (CFD) modeling are complementary tools for the development of thermal analysis, in-depth study of each reaction, and prediction of the gas flow to further anticipate problems that can occur during the combustion phase. The understanding of the processes in the fuel bed is rather limited, as it is difficult to obtain information through direct measurements because of the limited physical and optical accessibility inside a grate-fired boiler.
2. Thermal Analysis in Biomass Combustion
As it is impossible to maintain repeatable and fully controlled conditions, and to monitor all the dynamics involved in lab-scale experiments, it becomes necessary to perform investigations at a small scale. This should be adequate to provide a controlled environment, and large enough to define realistic conditions. Furthermore, due to the design and operation of industrial biomass boilers, there is a need to model the combustion to further determine their key operating and design parameters. Moreover, biomass combustion in grate-fired boilers can be described as a series of reactions, which begins at a relatively small scale.
As represented in Figure 1, a comprehensive understanding of devolatilization is fundamental to the conversion process. In order to completely characterize this stage, the kinetics of the reaction and the determination of the volatiles released are essential. The study of biomass combustion behavior and the kinetics of the solid-state reactions have been developed through fundamental thermal analysis methods. According to the International Confederation for Thermal Analysis and Calorimetry (ICTAC), thermal analysis is referred to as a group of techniques where a property of the sample is monitored against time and/or temperature and, consequently, the change of the sample in terms of its weight is measured as a result of an imposed temperature profile in a specified atmosphere [14]. In previous work, thermogravimetric analysis (TGA) is the most common thermoanalytical technique used for solid-phase thermal degradation studies and for kinetic measurements [15,16], while analysis of the gaseous release process and the heat and mass transfer effects can be evaluated using the same technique but at a larger scale, which is commonly known as macro-TGA [17].
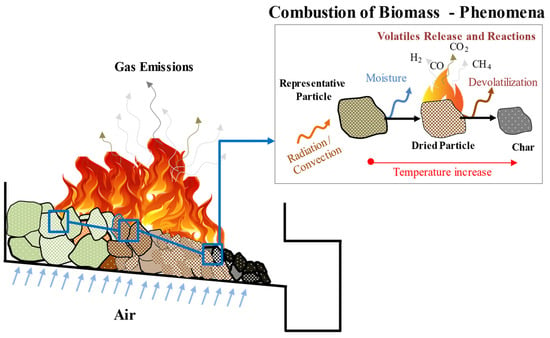
Figure 1. An overview of the phenomena occurring during the multi-scale biomass combustion process.
2.1. Thermogravimetric Analysis
As biomass combustion is a complex process [18], it is important to understand the physical and chemical processes involved at the particle level to enable proper understanding [19,20]. TGA is a powerful tool used to study the devolatilization rate during the biomass combustion process and obtain important parameters which are essential in characterizing and understanding its behavior [21,22]. TGA is widely implemented for investigating and comparing thermal degradation events and kinetics during the combustion of solid materials such as coal and biomass [23]. The decrease in mass is measured under controlled conditions while the thermal process is taking place, as the temperature increases with time. Consequently, information about the thermal conversion dependency on temperature will be obtained at the particle scale. According to the search results from the Web of Science Database using the keywords “thermogravimetric analysis” and “biomass”, there has been a growing trend around biomass and the application of TGA to biomass in scientific journals since 2000. Figure 2 presents the annual number of publications from 2000 to 2021, which highlights the use and importance of TGA to investigate the thermal decomposition of biomass.
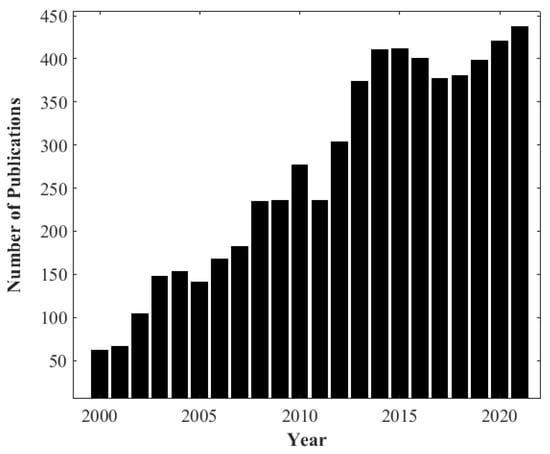
Figure 2. Number of annual publications from 2000 to the present on thermogravimetric analysis, TGA, and biomass: Data Source: results from Web of Knowledge Database.
A thermogravimetric analyzer consists of a sample pan that is supported by a precision balance placed in a furnace where the heating rate and environment can be controlled. Time, temperature, and weight are the three variables continuously measured and recorded during the TGA experiment. Taking the first derivative of such recorded data, known as derivative thermogravimetry (DTG), important parameters of thermal behavior characterization are provided. These key parameters are initial decomposition (Tin), peak (Tmax), and burnout (Tb) temperatures. Tin corresponds to the beginning of the weight loss, and it is defined as the temperature at which the rate of weight loss reaches 1%/min after the initial moisture loss peak in the DTG profile. Tmax is the point at which the maximum reaction rate occurs. Tb is identified when the last peak comes to the end and the temperature at which the sample is completely oxidized. It is taken as the point immediately before the reaction ceases when the rate of weight loss is down to 1%/min [24]. All this information allows for the thermal decomposition characterization of biomass samples and, in particular, the last two characteristic temperatures are important fuel parameters, especially in establishing the residence time in the combustion chamber. The experimental procedure and the most important points are illustrated in Figure 3.
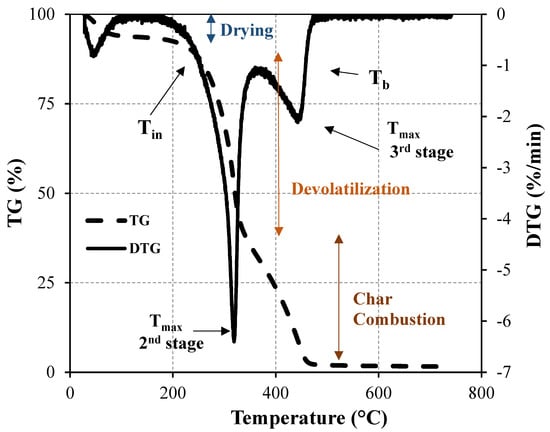
Figure 3. Mass loss as a function of time (above) and temperature (below) during the combustion of biomass [25].
The shape of the thermogravimetric curves (TG) and DTG curves is dependent on several factors, including the type of biomass; atmosphere and its flow rate; the heating program which includes the heating rate and the final temperature; initial mass; and the particle size of the fuel [26]. These constitute the main factors that affect the TG and that will determine the characteristic thermal decomposition behavior. The initial mass and particle size should be as small as possible to avoid the effect of heat and mass transfer limitations [11,27]. Regarding the atmosphere, there are two options to be considered: the oxidative or inert. An oxidative atmosphere greatly affects the devolatilization behavior [28]. The final temperature must be high enough for the complete decomposition of the carbonaceous materials. Finally, the heating rate is an important parameter as it greatly affects the rate of release of the volatiles due to the thermal inertia of the particles, and, in this way, different heating rates should be applied in order to study its influence [29].
Non-isothermal experiments are generally adopted for the determination of kinetic parameters as they are considered more reliable and less time-consuming when compared with isothermal experiments [30]. Moreover, TGA is very useful in studying the kinetics of biomass combustion because it is a simple and effective way to obtain information on the processes taking place for determining the kinetic parameters [31,32,33,34]. Thus, non-isothermal experiments include information on the temperature dependence of the reaction rate, and it is commonly believed that this would be sufficient to derive Arrhenius parameters and the reaction model of a process [35]. Consequently, the heating rate is one of the most relevant parameters in TGA as it affects thermal decomposition and, usually, experiments need to be performed with different heating rates to resolve possible compensation effects [36]. Thus, the Kinetics Committee of the ICTAC recommends that no less than three different temperature programs should be applied to obtain quality kinetic data [37].
The TGA results depend on several factors, but studies with a low heating rate are better at determining more precisely the temperatures from which the pyrolytic reactions start and avoiding transport effects [38]. In addition to the influence of the temperature and heating rate, Williams et al. [39] showed that the composition of the final products is dependent on the atmosphere. According to Vamvuka et al. [40], decreasing the oxygen concentration and increasing the particle size and moisture content will cause the ignition and burnout temperature to increase. Consequently, these variations will increase the residence time in the combustion chamber. Furthermore, Vamvuka et al. [40] reported that the composition of the gases, tars, and chars derived from biomass combustion depends on the heating rate and final temperature amongst other factors. Mani et al. [41] also investigated the influence of different parameters and found that the curves corresponding to the third stage of pyrolysis depend upon the particle size, initial weight, and heating rate of the pyrolysis process. Furthermore, an increase in the particle size and heating rate increases the char yield at the end of the experiments. Boriouchkine et al. [42] investigated the combustion of different particle sizes of spruce bark and wood residues. This study revealed that larger particles produced the highest maximum mass-loss rate when compared to smaller particles. Regarding the effect of the heating rate, Yorulmaz et al. [43] analyzed the combustion kinetics of treated and untreated waste wood using TGA under three different heating rates. This study revealed that by increasing the heating rate, the peak and burnout temperatures for all the samples were also increased, and higher temperatures were detected for the same weight loss. Shen et al. [44] examined the effect of the heating rate of four different biomass species, and the experimental results were used to develop a two-step reaction kinetic scheme with the activation energy depending on the heating rate. There have also been some studies that analyzed the effect of different heating rates on biomass decomposition under inert atmospheres [30,45,46,47,48,49,50]. However, as reported by Shen et al. [51] and Anca-Couce et al. [52], the presence of oxygen enhances biomass decomposition and promotes char combustion. Furthermore, the kinetic parameters derived from oxidant environments differ remarkably from experiments in the absence of oxygen [53].
Furthermore, in a real application of biomass combustion to produce power, for instance in an industrial grate-fired boiler, the temperature of the biomass increases and volatiles are quickly released due to the high heating rate that they are exposed to (around 1 to 100 K/s) [54]. This fast reaction results in insufficient air diffusing into the biomass, and ambient oxygen concentration varies over time which means that the reaction will change from pyrolysis to combustion [55]. Although modern boilers operate with oxygen-limited combustion under a low primary air flow rate, it is important to point out that most of the time this equipment operates with reaction-limited combustion due to a high primary air supply [56]. However, most of the studies in the literature have investigated pyrolysis using inert atmospheres [40,44,46,47,57,58,59,60,61,62,63,64].This is due to the fact that pyrolysis is the first step in thermochemical processes such as combustion and gasification [64].
Few results have been generated from experiments with air [36,43,44,65,66,67,68]. Shen et al. [51] and Anca-Couce et al. [52] reported that the presence of oxygen enhances biomass decomposition and promotes char combustion. Furthermore, the kinetic parameters resulting from oxidative atmospheres differ significantly from those in experiments conducted in the absence of oxygen [52]. Therefore, to simulate combustion conditions, it is important to study thermal behavior and kinetics in an oxygen atmosphere. Thus, in order to understand these differences, the influence of both oxidative and non-oxidative atmospheres on biomass thermal conversion have been studied by different authors [24,51,69,70]. Munir et al. [69] analyze the thermal characteristics of four waste biomass materials and the results showed that it is a complex phenomenon due to different microstructural and elemental characteristics as well as the type of atmosphere. The authors found that the weight loss rate in an inert atmosphere was slower, and its reactivity was 52% to 77% less than in oxidative conditions. Similar results were reported more recently by Sher et al. [70] who assessed the thermal and kinetic behaviors of diverse biomass fuels to provide valuable information for the power generation industry.
Yuzbasi et al. [24] compared the pyrolysis and combustion of co-firing biomass and coal with the individual behavior of each solid fuel. Regarding pyrolysis, a similar trend was obtained up to 700 °C. Furthermore, the oxygen levels shift the combustion profile to lower temperatures and increase the weight loss rate.
Shen et al. [51] investigated the thermal degradation of pine and birch and applied a new kinetic model, the distributed activation energy model (DAEM). DAEM was found unsuitable to describe the thermal decomposition of biomass under oxidant conditions due to the capacity of oxygen to accelerate the mass loss in the first stage and promote complex reactions in the second stage. Furthermore, some works analyzed the influence of oxidant and non-oxidant environments through experiments with different oxygen concentrations [28,55,71,72,73].
Fang et al. [55] studied the effects of oxygen concentration on the mass-loss rate and kinetics of pyrolysis and combustion of wood. The author stated that the mass-loss rates of wood were independent of oxygen concentration when the temperature was below 250 °C. Furthermore, it was found that the activation energy varied linearly with oxygen concentration at the first stage. Moreno et al. [73] also studied the kinetics of wood wastes and solid wood under different conditions examining three or four reactions with regards to whether the reaction occurred under oxidative or non-oxidative conditions. In turn, Amutio et al. [72] proposed a kinetic model consisting of six simultaneous reactions.
Chouchene et al. [71] studied the effect of three atmospheres with different oxygen content on the thermal degradation of solid waste. It was verified that pyrolysis under inert conditions takes place according to two different stages (drying and devolatilization) while under oxidative conditions a third stage, char oxidation, occurs. On the other hand, Su et al. [28] analyzed the effect of oxygen content on the thermal degradation of pine and similar results were obtained. The oxygen promoted the degradation of biomass, and a third stage was observed.
While the previous literature has cast light on TGA under a variety of conditions, only a few works have succeeded in analyzing thermal conversion, and determining all the kinetic parameters of experiments covering the possibility of oxidative and non-oxidative conditions with different flow rates. To date, several studies have considered these different parameters, including particle size [40,41,42] and heating rate [30,36,43,44,45,46,47,48,49,50,51,52,74,75,76,77,78,79,80], while studying their influence on thermal degradation behavior and kinetics. Additionally, in the literature, there are several kinetic data derived from the weight loss curves of biomass fuels in inert [29,41,42,46,47,48,50,60,61,62,79,81,82,83,84,85,86] and air [27,36,43,66,67,87,88,89] atmospheres. There are also other investigations that have studied the influence of the atmosphere and applied both atmospheres [24,28,51,55,69,70,73]. This extensive literature review concludes with the selection and analysis of works that analyzed the most representative solid biomass fuels (eucalyptus, acacia, and pine), which are summarized in Table 1.
Table 1. Literature review of experimental works that used pine, acacia, and eucalyptus samples.
| Author | Country | Reactor Model | Biomass Type | Surrounding Environment |
Mass (mg) |
Size (µm) |
Final Temperature (K) |
Heating Rate (K/min) | Kinetic Method |
|---|---|---|---|---|---|---|---|---|---|
| Xu et al. [90] 2021 | China | SDTA851E | Pine | Air 60 mL/min |
NA | <200 | 873 | 5 to 40 | 2-stage mechanism and OFW, Starink, DAEM, and CR |
| Chen et al. [91] 2020 | China | SDTA851E | Pine needle | Air 100 mL/min |
1.6 | 75–150 | 870 | 5 to 40 | 3-stage mechanism and OFW, KAS, and CR |
| Fu et al. [92] 2019 | China | TA Instrument SDT Q600 | Eucalyptus bark | N2 100 mL/min |
5–10 | 150–300 | 1073 | 10 to 30 | Model-fitting |
| Vega et al. [93] 2019 | Colombia | LINSEIS, STA PT-1600 | Pine and Acacia | N2/O2 mixture 50/13 mL/min |
10 | mesh 30 and mesh 60 | 1173 | 5 to 15 | OFW |
| Mishra et al. [30] 2018 | India | Hitachi, TA-7000 | Pine, sal sawdust, and areca nut husk | N2 50 mL/min |
8 | <1000 | 1173 | 5 to 25 | 1-global, KAS, OFW, CR, FR and DAEM |
| Wadhwani et al. [94] 2017 | Australia | Mettler Toledo TGA/DSC 1 | Pine and eucalyptus | N2 20 mL/min |
7.5 | 1–4000 | 1173 | 5 to 100 | 1-global, KAS and OFW |
| Cai et al. [95] 2016 | China | NETZSCH STA 409 PC | Eucalyptus and paper mill sludge |
Air 200 mL/min |
6 | <200 | 1223 | 10 to 40 | KAS and Starink |
| Álvarez et al. [74] 2016 | Spain | Perkin Elmer STA 6000 | 28 different biomass samples | Air 40 mL/min |
10 | 250–500 | 1173 | 5 to 20 | 2-stage reaction and KAS, OFW, CR |
| Yu et al. [75] 2016 | China | TA Instruments, SDT Q-600 | Eucalyptus bark | Air 100 mL/min |
10 | 200–600 | 1223 | 10 to 20 | 2-stage reaction and OFW and CR |
| Soria-Verdugo et al. [76] 2015 | Spain | TA Instruments Q-500 | Pine, olive kernel, thistle flower, and corncob | N2 60 mL/min |
10 | <100 | 1073 | 10 to 40 | DAEM |
| Soria-Verdugo et al. [76] 2015 | Spain | TA Instruments Q-500 | Pine, olive kernel, thistle flower, and corncob | N2 60 mL/min |
10 | <100 | 1073 | 10 to 40 | DAEM |
| Chen et al. [77] 2015 | China | Pyris1 TGA Instrument | Eucalyptus leaves, bark, and sawdust |
Ar 100 mL/min |
5 | 74 | 1073 | 5 to 50 | DAEM |
| Mishra et al. [49] 2015 | India | DTG-60 unit | Pine | N2 100 mL/min |
10 | 50 | 973 | 5 to 40 | OFW, KAS, FR, VY, VY AIC, and z(α) master plots |
| Saldarriaga et al. [63] 2015 | Spain | TA Instruments Q-500 |
Pine | N2 60 mL/min |
10 | <100 | 873 | 3 to 200 | DAEM |
| Soria-Verdugo et al. [78] 2014 | Spain | TA Instruments Q-500 |
Pine | N2 60 mL/min |
10 | <100 | 873 | 3 to 200 | DAEM |
| Fang et al. [96] 2013 | China | Mettler Toledo TGA/SDTA851 | Pine | Air 60 mL/min |
10 | <2000 | 773 | 30 | 1-global, CR |
| Anca-Couce et al. [52] 2012 | Germany | Linseis Thermal Analysis, L81/1000 | Pine | N2 and O2 | 2–4 | 200 | 873 | 2.5 to 10 | FR, KAS, and Fitting algorithm |
| Amutio et al. [72] 2012 | Spain | TA Instruments Q5000 | Pine | N2 and O2 100 mL/min |
10 | <200 | 1073 | 15 | Optimization model |
| Shen et al. [51] 2011 | United Kingdom | TGA Mettler Toledo TGA/SDTA 8951E | Pine | N2/Air 50 mL/min |
<5 | <300 | 1173 | 5 to 30 | 1-global, CR and DAEM |
| Kim et al. [45] 2010 | Republic of Korea | TA Instruments, Q-50 | Pine | N2 20 mL/min |
25 | 600 and 850 | 1073 | 5 to 50 | Differential method |
| Shen et al. [44] 2009 | United Kingdom | TGA Mettler Toledo TGA/SDTA 8951E | Pine | Air 60 mL/min |
<5 | 500 | 1073 | 5 to 50 | 2-stage reaction, CR |
| Lapuerta et al. [97] 2007 | Spain | TGA Seiko Instruments 6200 | Pine | N2 100 mL/min |
10 | <50 | 1100 | 5 to 40 | Fitting algorithm |
| Lapuerta et al. [98] 2004 | Spain | TGA Seiko Instruments 6200 | Pine | N2 50 mL/min |
10 | <500 | 1100 | 10 to 60 | Fitting algorithm |
| Gronli et al. [59] 2002 | Norway | TA Instruments SDT 2960 | Pine | N2 150 mL/min |
5 | NA | 773 | 5 | Optimization model |
| Bilbao et al. [65] 1997 | Spain | SETARAM 92 | Pine | Air 100 mL/min |
3 and 20 | 630 | ≈1023 | 7 and 12 | NA |
CR—Coats Redfern. DAEM—Discrete Activation Energy Model. KAS—Kissinger-Akahira-Sunose. NA—Not Available. OFW—Ozawa-Flynn-Wall. TA—Thermogravimetric Analyzer. Vy—Vyazovkin.
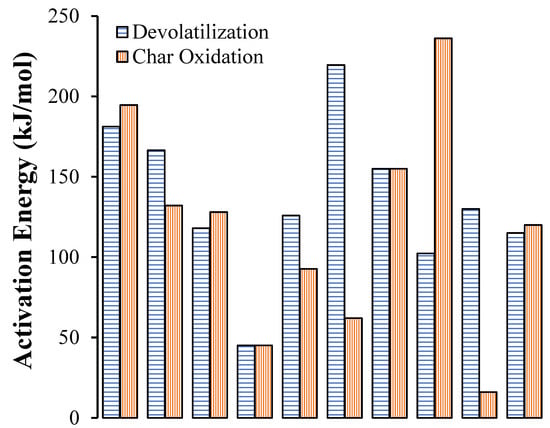
Hence, considering the current state-of-the-art in this subject, many factors can affect the kinetic parameters, including not only the process conditions, heterogeneity of the sample, heat and mass transfer limitations, and systematic errors, but also the processing and method for the analysis of the TGA results [16]. Consequently, a wide range of kinetic parameters has been reported in the literature and, therefore, direct comparisons are not possible. As evidence of this inconsistency, Figure 4 presents the different activation energy values reported in the literature for pine devolatilization and char oxidation. The set of results show a wide dispersion of data, which can be by a factor of up to five, depending on the author. This clearly shows that further experimentation should be carried out using TG analysis for the determination of the activation energy at each step of the biomass conversion.
2.2. Macro Thermogravimetric Analysis
Experiments in a lab-scale reactor are an interesting alternative to address the biomass conversion in a real scale, and also to provide complementary knowledge to the TGA results about the kinetics of the reaction in the thermal biomass decomposition. This is also particularly interesting because the conditions in biomass industrial boilers are different, and it is important to investigate the thermal decomposition of larger particles and higher biomass quantities than those possible with TGA experiments. This possibility will allow us to take into consideration the heat and mass diffusion in the reaction mechanism. Furthermore, using larger samples, the effects of secondary reactions together with the possibility of operating at higher heating rates can also affect the reaction kinetics. Consequently, due to the complexity of the combustion process inside industrial boilers, which is enhanced with the motion of the fuel bed, there are several authors that report experiments in a batch reactor in order to describe the entire process in a traveling or vibrating grate boiler [100,101,102,103,104,105]. These experiments often include the combustion of a large amount of biomass (in the range of a number of kilograms). Experiments using batch reactors are also performed to quantify the implications of differences in fuel properties, and to investigate the propagation of a combustion front, i.e., the drying, pyrolysis, and char combustion process, in a bed of biomass particles. Such experiments are useful to develop parametric studies with different operating conditions (primary and secondary air flow rate and temperature) and different fuel properties (moisture content, volatile matter, ash content, chemical composition, heating values, and particle size). The ignition front velocity, ignition rate, conversion rate, and temperature of the reaction zone are the parameters most commonly obtained to evaluate combustion behavior.
This entry is adapted from the peer-reviewed paper 10.3390/en16186705
This entry is offline, you can click here to edit this entry!
