Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Worldwide, oesophageal cancer is the sixth leading cause of deaths related to cancer and represents a major health concern. Sub-Saharan Africa is one of the regions of the world with the highest incidence and mortality rates for oesophageal cancer and most of the cases of oesophageal cancer in this region are oesophageal squamous cell carcinoma (OSCC). The development and progression of OSCC is characterized by genomic changes which can be utilized as diagnostic or prognostic markers.
- tumor mutational burden
- HPV
- HIV
- miRNA
- alternative splicing
1. Introduction
In most patients, oesophageal cancer is characterized by late presentation, resulting in poor outcomes. Patients tend to only consult their healthcare practitioners at these later stages due to a variety of factors. These include no noticeable symptoms during the early stages of the disease and a lack of biomarkers for early detection (Reviewed in [1]). Due to this lack of biomarkers, diagnosis currently relies on well-established methods of histology followed by staging imaging prior to planning any treatment (Reviewed in [2]). A further group of complications that is especially prevalent in developing countries arises due to challenges within the health care system structures. These can include poor referral patterns and existing inequalities within the healthcare system. Several studies have tested different proteins and genetic markers for their potential use as biomarkers to improve current methods for the diagnosis of oesophageal carcinoma [3][4]. However, the advances in sequencing, along with increasing numbers of genome-wide association studies (GWAS) and the application of this genomics data acquisition methods to cancer, has started to change this; this may potentially lead to early detection and the promise of precision oncology. Not only do these studies have the potential to uncover biomarkers that may potentially be useful in the early diagnosis and treatment of oesophageal cancer, but they can be used to characterize populations that have previously been neglected in genomic studies, such as Africans. Next-generation sequencing (NGS) of tumor-derived DNA and RNA can reveal multiple cancer-specific changes to the genome and transcriptome. Sequence mutations, insertions, deletions, rearrangements, copy number variations (CNVs), and loss of heterozygosity can all be revealed by sequencing DNA isolated from cancer cells. Sequencing of the transcriptome of cancer cells can reveal the presence of, gene fusions, alternately splice mRNA transcripts and changes in the transcript levels of mRNAs (coding genes) or small non-coding RNAs. This would give information concerning gene or non-coding RNA transcription profiles which would be specific to a particular type or stage of cancer [5].
The use of NGS in large-scale cancer genomics discovery projects has resulted in the elucidation of the underlying molecular basis and mechanisms for cancer development and progression in a variety of tissues, including the genetic drivers of cancer [5]. These cancer-specific molecular changes, whether they are gnomic, transcriptomic or epigenomic, may serve as useful biomarkers. This can only happen once they have been identified as being associated with diseased tissues and not to be present in in normal tissue [6]. These changes, therefore, hold the promise of serving as new diagnostic and prognostic tools and may complement or replace histological analysis in this regard [3].
2. Altered Gene Expression in OSCC
Oesophageal cancer develops from stratified squamous epithelium and is associated with molecular abnormalities in a variety of genes. These include structural chromosomal abnormalities, gene upregulation or downregulation, somatic pathogenic variations, and hyper-methylation (Figure 1). Oesophageal cancer generally develops in response to a chronic inflammatory insult to the normal cells which results in somatic mutations, CNVs and chromosomal aberrations. These changes result in cancer formation and progression as normal epithelium undergoes basal cell hyperplasia and intraepithelial neoplasia (dysplasia) to give rise to invasive carcinoma [7][8][9].
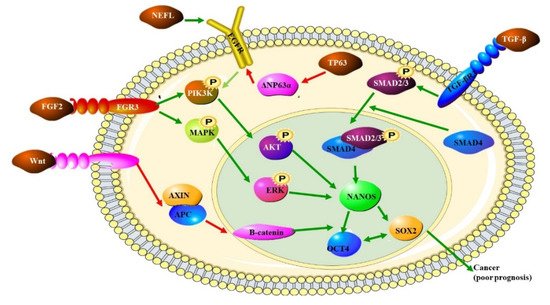
Figure 1. The role of various pathways in OSCC: the transcription factor SOX2, the ligand for the FRIZZLED receptor, WNT and the transcriptional activator TP63 are all up regulated in OSCC. The increased expression of these proteins also indicates a poor prognosis. SOX2 forms a trimeric complex with OCT4 on DNA and NANOS which controls the expression of several genes involved in proliferation. NANOS is a translational repressor which leads to the increased expression of metalloproteinases. Increased WNT expression leads to increased levels of β-catenin. TP63 inhibits the production of ΔNP63α, which dampens PIK3K by preventing EGFR signaling [10].
Next-generation sequencing as a method to characterize genomic changes in cancer tissues offers the advantage of not having to have any prior knowledge of the DNA sequences being studied. NGS can be used to analyze the whole genome, whole exome or known cancer-causing genes using targeted cancer panels. These analyzes have detected widespread genomic alterations in OSCC [11]. The genomic characterization of tumors allows for specific molecular changes to be targeted for the development of unique therapies and interventions for each type of cancer, according to the underlying biological basis of each type of cancer. This allows scientists to use these genomic changes as biomarkers to monitor the drug response of the disease and identify the initiation and mechanisms underlying drug resistance. This information would allow for guided clinical decision making regarding the treatment of patients [5].
3. Tumor Mutational Burden
Cancer is the phenotypic endpoint of accumulated genetic and epigenomic alterations. A metric was created to quantify the number of mutations present in a tumor. Metric is known as the tumor mutation burden (TMB) [12] and is calculated by dividing the number of missense mutations in the tumor genome by the size of the of the DNA being measured, in megabases. Normally this is by either the size of the protein coding genes which is 35–45 Mb in humans [13], or by the entire genome, 3.3 Gb in humans. The former is used for whole exome sequencing (WES) and the latter for whole-genome sequencing (WGS) [14].
The TMB is also expressed as the mutations per million bases of the tumor, genome examined [15][16]. TMB is a continuous variable, ranging from 0.001 mut/Mb to more than 1000 mut/Mb as has been observed across and within cancer types [13]. It is speculated that a tumor bearing a higher TMB level may be highly immunogenic as it is likely to harbor more neoantigens. These neoantigens can then be targeted to a higher degree by activated immune cells capable of inducing an anti-tumor immune response, which would result in a good clinical prognosis [12][17]. This increase in neoantigens occurs because TMB is believed to be a good indicator of the number of immunogenic neopeptides displayed on the surface of tumor cells, which influences patient response to ICIs [13].
The Checkmate trials are a clinical trial of the adjuvant checkpoint inhibitor Nivolumab in high-risk bladder cancer that has invaded muscle-tissue [18]. According to the Checkmate trials, allowed the researchers to establish a TMB threshold for a high TMB is ≥10 mutations per megabase (10p6) (mut/Mb) and was demonstrated as a robust, independent biomarker of response based on the objective response rates of the tumors in those studies not improving much beyond this threshold [12][14]. Although TMB—H prevalence varies widely between different types of tumors, it has emerged as a potential biomarker for tumors that are likely to respond to immune checkpoint inhibitor therapy [19].
In oesophageal cancer, it was found that clinical factors, such as age, gender, tumor grade, tumor stage, race, and prior radiation treatment, were not associated with TMB levels and there was no significant correlation between TMB and TNM stages. A multivariate regression analysis of TMB levels and OSCC associated risk factors, indicated that TMB is a risk-independent prognostic factor, with a lower TMB being associated with a better prognosis [12]. Oesophageal adenocarcinoma (OAC) has a higher TMB than OSCC. A study comparing the two have found a mean TMB-High >17 mut/Mb in 3% of OSCC were compared to 8% for OAC [7][13].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10102359
References
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Prim. 2017, 3, 17048.
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.M.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Hussain, S.; Kibiki, G.; et al. A multinational review: Oesophageal cancer in low to middle-income countries. Oncol. Lett. 2020, 20, 42.
- Kaz, A.M.; Grady, W.M. Epigenetic biomarkers in esophageal cancer. Cancer Lett 2014, 342, 193–199.
- Kunzmann, A.T.; McMenamin, Ú.C.; Spence, A.D.; Gray, R.T.; Murray, L.J.; Turkington, R.C.; Coleman, H.G. Blood biomarkers for early diagnosis of oesophageal cancer: A systematic review. Eur. J. Gastroenterol. Hepatol. 2018, 30, 263–273.
- Berger, M.F.; Mardis, E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365.
- El-Deiry, W.S.; Goldberg, R.M.; Lenz, H.-J.; Shields, A.F.; Gibney, G.T.; Tan, A.R.; Brown, J.; Eisenberg, B.; Heath, E.I.; Phuphanich, S.; et al. The current state of molecular testing in the treatment of patients with solid tumors, 2019. CA Cancer J. Clin. 2019, 69, 305–343.
- Chen, X.X.; Zhong, Q.; Liu, Y.; Yan, S.M.; Chen, Z.H.; Jin, S.Z.; Xia, T.L.; Li, R.Y.; Zhou, A.J.; Su, Z.; et al. Genomic comparison of esophageal squamous cell carcinoma and its precursor lesions by multi-region whole-exome sequencing. Nat. Commun. 2017, 8, 524.
- Walker, R.C.; Underwood, T.J. Molecular pathways in the development and treatment of oesophageal cancer. Best Pract. Res. Clin. Gastroenterol. 2018, 36–37, 9–15.
- Walline, H.M.; Carey, T.E.; Goudsmit, C.M.; Bellile, E.L.; D’Souza, G.; Peterson, L.A.; McHugh, J.B.; Pai, S.I.; Lee, J.J.; Shin, D.M.; et al. High-Risk HPV, Biomarkers, and Outcome in Matched Cohorts of Head and Neck Cancer Patients Positive and Negative for HIV. Mol. Cancer Res. 2017, 15, 179–188.
- Gen, Y.; Yasui, K.; Nishikawa, T.; Yoshikawa, T. SOX2 promotes tumor growth of esophageal squamous cell carcinoma through the AKT/mammalian target of rapamycin complex 1 signaling pathway. Cancer Sci. 2013, 104, 810–816.
- Di Resta, C.; Galbiati, S.; Carrera, P.; Ferrari, M. Next-generation sequencing approach for the diagnosis of human diseases: Open challenges and new opportunities. EJIFCC 2018, 29, 4–14.
- Yuan, C.; Xiang, L.; Cao, K.; Zhang, J.; Luo, Y.; Sun, W.; Zhang, N.; Ren, J.; Zhang, J.; Gong, Y.; et al. The prognostic value of tumor mutational burden and immune cell infiltration in esophageal cancer patients with or without radiotherapy. Aging 2020, 12, 4603–4616.
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825.
- Pestinger, V.; Smith, M.; Sillo, T.; Findlay, J.M.; Laes, J.-F.; Martin, G.; Middleton, G.; Taniere, P.; Beggs, A.D. Use of an Integrated Pan-Cancer Oncology Enrichment Next-Generation Sequencing Assay to Measure Tumour Mutational Burden and Detect Clinically Actionable Variants. Mol. Diagn. Ther. 2020, 24, 339–349.
- Moody, S.; Senkin, S.; Islam, S.M.A.; Wang, J.; Nasrollahzadeh, D.; Penha, R.C.C.; Fitzgerald, S.; Bergstrom, E.N.; Atkins, J.; He, Y.; et al. Mutational signatures in esophageal squamous cell carcinoma from eight countries with varying incidence. Nat. Genet. 2021, 53, 1553–1563.
- Sawada, G.; Niida, A.; Uchi, R.; Hirata, H.; Shimamura, T.; Suzuki, Y.; Shiraishi, Y.; Chiba, K.; Imoto, S.; Takahashi, Y.; et al. Genomic Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology 2016, 150, 1171–1182.
- Zhang, R.; Hu, Y.; Zhou, T.; Han, W.; Liu, Y.; Qian, J.; Chen, Y.; Liu, X.; Liu, S.; Yu, Y.; et al. The mutation profiles of cell-free DNA in patients with oesophageal squamous cell carcinoma who were responsive and non-responsive to neoadjuvant chemotherapy. J. Thorac. Dis. 2020, 12, 4274–4283.
- Kumar, N. Checkmate 274 trial: Is Nivolumab the new standard in adjuvant setting for high-risk muscle invasive urothelial carcinoma? Indian J. Urol. 2021, 37, 369–371.
- Shao, C.; Li, G.; Huang, L.; Pruitt, S.; Castellanos, E.; Frampton, G.; Carson, K.R.; Snow, T.; Singal, G.; Fabrizio, D.; et al. Prevalence of High Tumor Mutational Burden and Association with Survival in Patients with Less Common Solid Tumors. JAMA Netw. Open 2020, 3, e2025109.
This entry is offline, you can click here to edit this entry!
