Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Latent tuberculosis infection (LTBI) represents a subclinical, asymptomatic mycobacterial state affecting approximately 25% of the global population. The substantial prevalence of LTBI, combined with the risk of progressing to active tuberculosis, underscores its central role in the increasing incidence of tuberculosis (TB). Accurate identification and timely treatment are vital to contain and reduce the spread of the disease, forming a critical component of the global strategy known as “End TB.”
- diagnostic
- Mycobacterium tuberculosis
- Latent tuberculosis infection
1. Introduction
Tuberculosis (TB) is caused mainly by Mycobacterium tuberculosis (MTB), an actinomycete closely related to saprophytic bacteria such as Mycobacterium smegmatis. Unlike most Gram-positive bacteria, MTB bacilli increase their peptidoglycan wall with a wide range of complex lipidoglycans, and this characteristic has posed significant challenges for the treatment and eradication of the disease. Despite continuous efforts to control and prevent TB, it remains a significant burden on global public health [1].
According to the 2022 report from the World Health Organization [2], 25% of the global population is infected with a latent form of tuberculosis (LTBI). This carries a lifetime risk of progressing to active disease ranging between 5% and 10%. The report also highlights that the primary focus in terms of diagnosis and treatment is currently on children and young adolescents (under 15 years of age), who account for approximately 11% of the global burden. This equates to an annual incidence of 1.1 million children contracting the disease. It is particularly alarming that nearly half of these are under five years of age, a demographic in which the mortality rate approaches 80%. Furthermore, adolescents aged 15 to 19 years represent another significant segment of infection, with over half a million active cases reported each year.
TB is primarily transmitted through microdroplets expelled by actively infected individuals when coughing, sneezing, or talking. These droplets can remain suspended in the air and infect nearby individuals [3]. The first line of defense against MTB is the respiratory mucosa, which contains a range of protective molecules such as immunoglobulins A, antimicrobial peptides (AMPs), antibodies, cytokines, and chemokines. When these molecules fail to control the invasion in the first instance, MTB disseminates to the pulmonary alveoli, and shortly thereafter alveolar macrophages, dendritic cells, and neutrophils are activated to attempt to eradicate the infection [4]. MTB has evolved and developed the ability to internalize macrophages to live within phagosomes, thereby overcoming nutrient availability and stressful environmental factors, while providing protection against drugs and therapeutic doses (Figure 1) [5].
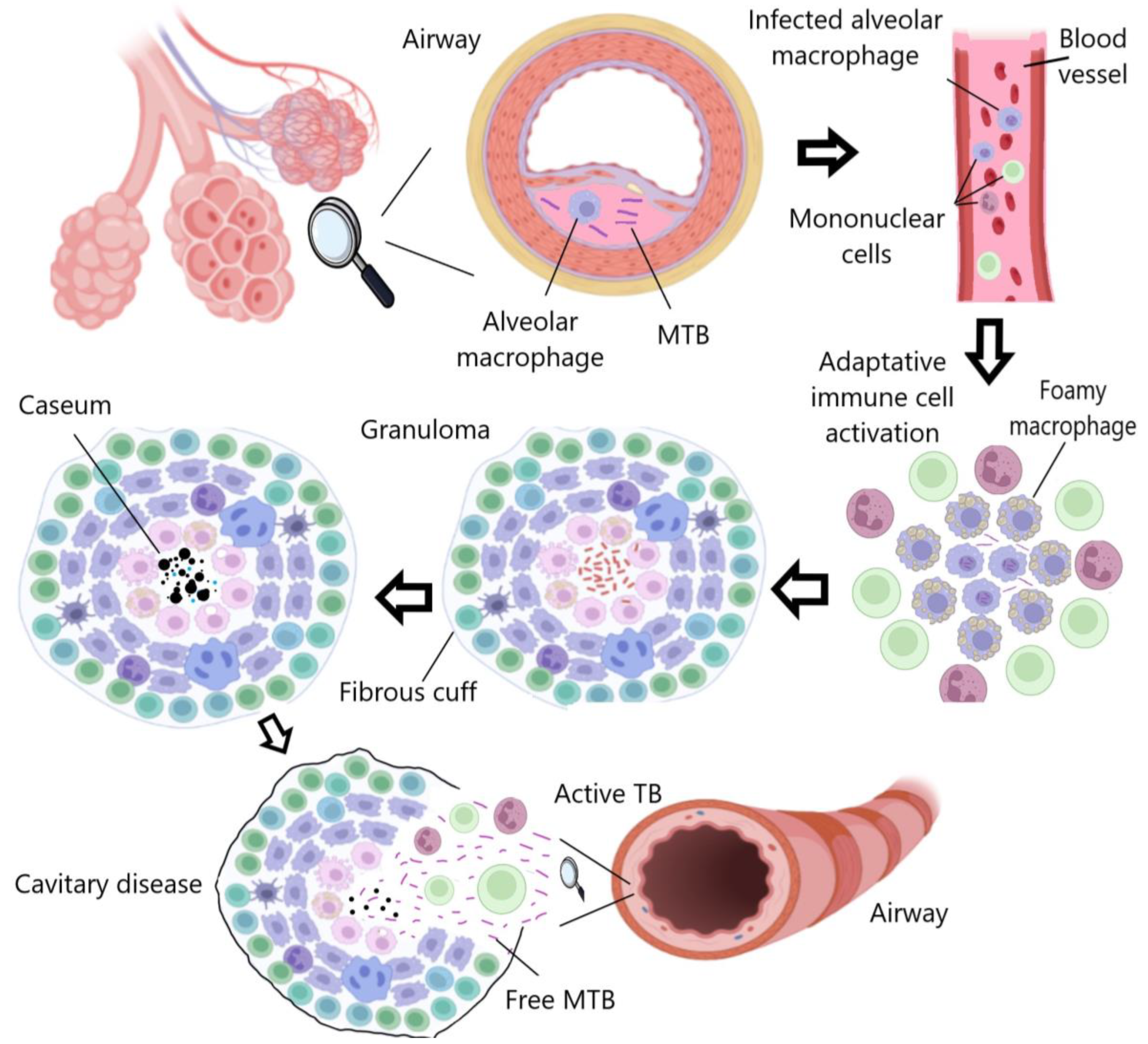
Figure 1. TB progression: Macrophages attempt to eradicate MTB, which persists intracellularly and induces an inflammatory response, leading to granuloma formation. The infection may become latent or progressive, resulting in cavity rupture and bacterial dissemination.
Among the people infected with MTB, there is a group of patients who develop an inactive form of TB (latent TB). MTB remains inactive, causing recurrent or chronic infections depending on the host’s immunological conditions [5][6]. Macrophages generally produce AMPs, cytokines, reactive oxygen species (ROS), and nitric oxide (NO) to eliminate pathogens [7]. However, MTB can inhibit these intracellular immune response mechanisms by promoting the production of IL-10. Prolonged exposure to this cytokine also affects the apoptosis, phagocytosis, autophagy, and Th1 immune response of myeloid cells [8]. All these functions are mediated by IL-10, as it binds to its receptor to activate STAT3, a protein that stimulates the transcription of specific genes that transcriptionally repress proinflammatory cytokine genes [9]. Additionally, proinflammatory cytokines are important for the retention of lymphocytes in non-lymphoid sites after MTB infection [10].
Latent tuberculosis infection (LTBI) is defined by the World Health Organization [11] as a “state of persistent immune response to stimulation by MTB antigens with no evidence of clinically manifest active TB”. It is triggered by a sustained immune response against MTB antigens and self-healing lesions that form granulomas, which act as a type of bacterial reservoir. Patients with LTBI are asymptomatic; they are infected with MTB but do not develop active TB disease. Furthermore, patients with LTBI are not infectious and cannot transmit TB infection to others [12]. Although TB is not a new disease, incidence rates are still high, and concern is growing due to the lack of a specific treatment to combat LTBI, which is difficult to treat due to the disease’s physiopathological characteristics and its challenging diagnosis [13].
2. Epidemiology of Latent Tuberculosis Infection
Roughly two decades ago, it was estimated that a third of the global population had LTBI. However, demographic changes, advancements in pharmacology, and enhanced TB control strategies have significantly revised this estimate. Currently, it is believed that 25% of the global population carries this latent infection [14]. The importance of controlling and preventing LTBI lies in its worldwide distribution and its high mortality rate when progressing to active disease, which caused 1.3 million recorded deaths in 2020, nearly twice the number of deaths caused by HIV [15]. Houben and Dodd [16] used mathematical models to estimate the global incidence of LTBI. Their findings indicated that, in 2014, 23% of the global population (approximately 1.7 billion people) was infected, with Southeast Asia, the Western Pacific, and Africa being the regions with the highest prevalence, representing approximately 80% of the global burden.
These findings are in agreement with the most recent update by Cohen et al. [17] on the prevalence of LTBI burden in a meta-analysis study based on the results of the interferon-γ release assays (IGRAs) and the tuberculin skin test (TST). This study reported a global prevalence of LTBI of 24.8% (95% confidence interval [CI 95%]: 19.7–29.9%) and 21.2% (CI 95%: 17.9–24.4%) for IGRAs and TST, respectively. However, it is important to emphasize that obtaining an accurate estimate of the LTBI rate remains a significant challenge due to the lack of a uniform standard for identifying this condition. China and India are the countries with the highest prevalence of LTBI, with approximately 350 million infected individuals in China [18] and an infection rate in India ranging between 40% and 50% in various populations, implying that more than 500 million inhabitants may have LTBI [19]. In the United States, it is estimated that approximately 13 million people have LTBI [20], with 80% of TB cases resulting from untreated latent infection [21]. In Africa, infection rates vary between 31.2% in Ethiopia, 49% in Uganda, and 55.2% in South Africa, with high prevalence in at-risk populations such as miners (89%) and healthcare workers (62–84%) in high-incidence areas. Between 5% and 15% of individuals with LTBI progress to active TB, with a higher risk associated with impaired immunity [22].
In the WHO European Region, the prevalence of LTBI is estimated at 13.7%, with 0.3% being recent infections at a higher risk of progressing to active TB. In low-incidence countries, most TB cases are generated through reactivation of LTBI acquired abroad due to global migration (Figure 2). Therefore, a significant challenge for European control programs is to establish a programmatic approach to LTBI management [23]. Despite the decrease in the global burden of LTBI, declining from one third to one quarter of the world’s infected population, significant challenges persist. Until 2015, there were 19.1 million people reported to be latently infected with high-risk multidrug-resistant TB (MDR-TB). MDR strains accounted for 1.2% of the overall LTBI burden and 2.9% of the burden among children under 15 years of age [24]. Currently, these numbers are increasing due to complications in lung diseases caused by the COVID-19 pandemic, posing serious challenges for LTBI management, a cornerstone of TB elimination strategies. Concerted efforts must be made globally to address the prevention and treatment of LTBI, with an emphasis on high-prevalence areas and at-risk populations, in order to achieve WHO’s objectives and reduce the global burden of this devastating disease [25].
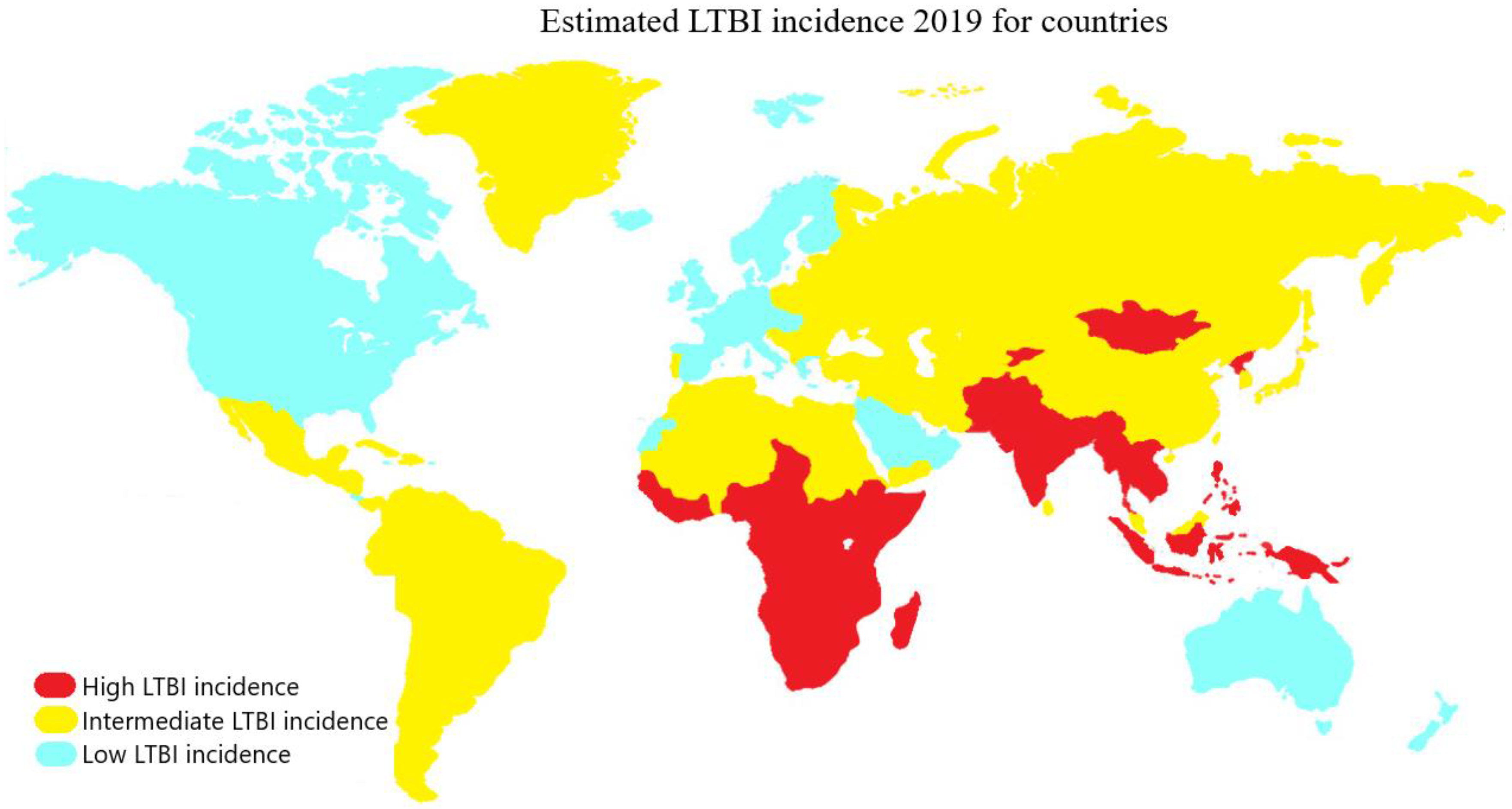
Figure 2. Countries with LTBI incidence are classified according to their prevalence into high (red), intermediate (yellow), and low (light blue) categories, corresponding to prevalence ranges of 28–36%, 19–20%, and 3–5%, respectively.
3. New Advances in The Diagnosis of LTBI
Two methods have been described as the most commonly used in the diagnosis of TB and probable screening tests for LTBI. One diagnostic method is the tuberculin skin test (TST), which is based on a purified protein derivative (PPD) of proteins secreted by MTB. This solution is injected intradermally into the patient’s forearm, aiming to induce a delayed hypersensitivity reaction caused by the T lymphocytes that will generate local inflammation mediated by various cytokines within a period of 48 to 72 h [26][27][28]. However, a limitation of the clinical application of this test is that the TST contains a mixture of non-specific antigens for MTB, which can result in false-positive results in patients previously vaccinated with Bacillus Calmette-Guerin (BCG) or patients who have been infected by non-tuberculous mycobacteria. On the other hand, false-negative results may occur in immunocompromised patients with insufficient T cell responses to generate the local hypersensitivity reaction [29][30][31].
In the quest for better specific tests that can more accurately identify the development of active TB in patients, interferon gamma release assays (IGRAs) have been developed. This in vitro blood analysis is based on T-cell-mediated immunity to specific antigens of MTB. The test is considered sensitive and specific for active TB and LTBI, as it was developed from two MTB-specific T-cell antigens: ESAT-6 and CFP-10 [32][33]. However, like the TST, this test is limited by its inability to differentiate active TB from LTBI. As a result, alternative detection tests are being sought or combinations of these. In the context of LTBI diagnosis in patients with inflammatory bowel disease undergoing immunosuppressive therapy (IST), Park et al. [34] suggest that the optimal diagnostic strategy is the combination of IGRA followed by TST. This sequence significantly improves accuracy in detecting LTBI, particularly in situations after IST initiation and in populations previously vaccinated with BCG. On the other hand, sole reliance on IGRA, especially in patients under IST, represents a less reliable approach; even with a negative IGRA result, there remains an underlying risk of tuberculous reactivation. This can be attributed to the ability of IST to decrease the positivity rate of IGRA. Additionally, the variability in TST efficacy, depending on the chosen cutoff value, and the potential drop-in positivity rates with fixed cutoffs during IST, underscore the necessity for a combined approach. It is worth noting that there have been documented cases of progression to active tuberculosis despite a negative IGRA outcome, emphasizing the significance of a thorough diagnostic strategy. On the other hand, Kang et al. [35] report that the combination of IGRAs and complete blood count (CBC) analysis was investigated as a possible method for differentiating active TB from LTBI. A total of 126 samples (blood, serum, and plasma) were used, and the authors concluded that the statistically most significant blood biomarkers in comparing their expression levels were total white blood cells, neutrophils, lymphocytes, and monocytes. However, further studies with a larger number of clinical samples are needed to confirm the importance of these biomarkers and improve the accuracy in differentiating MTB pathological states.
The pace of development of more accurate tests for LTBI has been slow. In 2001, the FDA approved a first-generation test, the QuantiFERON-TB. Subsequently, in 2005, a second generation of this test, known as QuantiFERON-TB GOLD in Tube (QFT), was approved. In 2008, another test, the T-SPOT.TB, was also approved. These tests are based on the IGRA. Today, these IGRAs are commercially available and are endorsed by Health Canada. Additionally, they carry the CE (Conformité Européenne) mark, certifying their use in Europe, and have received the exclusive endorsement of the World Health Organization for their implementation [28][36].
The QFT assay is an enzyme-linked immunosorbent assay (ELISA) that uses peptides from the RD1 ESAT-6 and CFP-10 antigens, targeting CD4 T-helper cell-mediated immune responses in a tube. Results are reported as quantification of IFN-γ [37]. There is currently a new generation of IGRA available, specifically the QuantiFERON-TB Gold Plus (QFT-Plus) assay. The difference with its predecessor lies in the addition of a new antigen specific to CD8 T cells in a second antigen tube (QFT-Plus Tube 2, TB2) specifically designed to stimulate CD8 and CD4 T cells. In this way, the new antigen tube complements the first antigen tube (QFT-Plus Tube 1, TB1) [38]. The importance of CD8 T cells in TB infection lies in their participation in the recognition and destructive attack on cells infected with MTB. They also have an effect during the replication of MTB in its active phase, which decreases during the treatment phase. Based on this evidence, a higher frequency of CD8 T cells is found in patients with active TB than in LTBI patients, indicating a direct relationship with antigenic load [39].
In 2018, Petruccioli et al. [40] evaluated the response to TB treatment in relation to the results of the QFT-Plus test. They contacted 74 participants in different stages of TB infection, including 46 participants with LTBI and 28 patients with active TB. The study showed that therapies can reduce the level of IFN-γ in response to stimulation by TB1 and TB2 peptides. However, this result varied greatly depending on various factors, the most impactful of which occurred in patients with clinical diagnosis, in comparison with those with microbiological diagnosis. This difference is likely due to the bacterial load maintaining a latent immune response, which is why the QFT-Plus test cannot yet be considered a definitive tool for differentiation and monitoring in the treatment of LTBI and active TB. On the other hand, the T-SPOT.TB test is an enzyme-linked immunospot assay (ELISPOT) performed on isolated and counted peripheral blood mononuclear cells (PBMCs) incubated with ESAT-6 and CFP-10 peptides. Results are reported as the number of IFN-γ-producing T cells (spot-forming cells). Inconclusive IGRA results may occur due to a low IFN-γ response to positive controls (mitogens) or a high background response to negative controls [36].
In order to compare the agreement and effectiveness of the two IGRAs (QFT-GIT and T-SPOT.TB) in the diagnosis of active TB, Du et al. [41] conducted a comparative study and concluded that both the sensitivity and specificity of T-SPOT.TB were slightly higher than those of QFT-GIT but did not reach statistical significance. This is likely due to the difference in the more complex technical characteristics involved in this test. When different groups were investigated, the concordances were 93.4%, 90.0%, and 93.7% in the confirmed TB, probable TB, and non-TB groups, respectively. Despite these results, no test is entirely suitable for the diagnosis of LTBI due to sensitivity and specificity issues, inability to distinguish infections with MDR-MTB strains, and the high costs involved.
In this line of research, concerning the development and optimization of different assays for the diagnosis and differentiation of TB and LTBI, surface markers on T cells were evaluated as a starting point, since these are the cells responsible for generating an immune response against MTB infection. Yang et al. [42] investigated the clinical utility of T cells expressing the CD161 marker in the effective differentiation of active TB. For this research, eight markers were selected using flow cytometry, with CD161 standing out as the promising marker for the differentiation of active TB from LTBI. Among the results obtained, it was observed that the percentage of T cells expressing CD161 was lower in active TB than in LTBI or healthy controls. However, these data were not sufficient, which is why the lymphocyte/monocyte ratio was included as a differentiation parameter, as these are also significantly reduced in an active TB infection. Although it was concluded that the CD161 marker measurement method has high specificity and sensitivity for differentiating active TB from LTBI, its main limitation in clinical application is that not all hospitals have flow cytometers.
As shown, most diagnostic tests are based on the immune response generated in peripheral blood. However, Das et al. [43] reported that MTB infection primarily targets the lung, highlighting the importance of exploring the pulmonary immune response, including alveolar T cells and alveolar macrophages. In addition, it has recently been demonstrated that DNA methylation is fundamental for the immune response, as confirmed by Karlsson et al. [44] who compared and evaluated epigenetic modifications in DNA methylomes in both alveolar macrophages and alveolar T cells. They identified a particular and different DNA methylation profile in patients without peripheral immune response who developed LTBI during the study. The differentially methylated genes (DMGs) identified in subjects who developed LTBI were overrepresented in the pentose phosphate pathway of alveolar macrophages and in IFN-γ signaling and migration in alveolar T cells. Finally, they argued that knowing the DNA methylation status in pulmonary immune cells can indicate who will develop LTBI. Another significant finding was that the identification of this methylation profile detects an early result of an LTBI infection, before any other available diagnostic method.
Similarly, Liu et al. [45] developed Genepop, a simple, rapid, and low-cost diagnostic method characterized by not requiring a cold chain, with a concordance of 91.6% based on loop-mediated isothermal amplification. This test is useful in domestic or field testing, and its importance lies in helping to prevent the spread of MTB in several countries due to its low production cost and easy operability. Despite the advances in developing new diagnostic methods for LTBI, MDR-TB strains have hindered the development of an effective and specific method for accurately diagnosing LTBI.
In pediatric populations, the challenge of diagnosing TB is notably accentuated. Traditional bacteriological methods often fall short due to the limited bacillary loads in children, leading to frequent misdiagnoses or confusions with other pediatric conditions. This diagnostic shortfall has contributed to a rising incidence and mortality from TB among the young. In light of this, starting in 2021, the World Health Organization has championed the integration of cutting-edge molecular diagnostic techniques (Table 1). These are designed to either supersede or complement existing methods, thereby amplifying the sensitivity and specificity of TB diagnosis in both children and adults [2][46].
Table 1. New diagnostic methods for the detection of TB. Data obtained from: The World Health Organization consolidated guidelines on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection, 2021 update.
| Test | Purpose | Application | Population | Principle |
|---|---|---|---|---|
| Xpert MTB/RIF | Detect MTB and RIF resistance | Pulmonary TB, extra-pulmonary TB, HIV co-infection | Adults and children | PCR |
| Xpert MTB/RIF Ultra | Detect MTB, minimize false RIF resistance results | TB meningitis, pulmonary TB, extra-pulmonary TB | Adults and children | PCR |
| Truenat MTB, MTB Plus, and MTB RIF Dx tests | Semi-quantitative detection of MTB complex, RIF resistance | Pulmonary TB, HIV co-infection | Adults | PCR |
| TB-mediated isothermal DNA amplification (LAMP) | Detect MTB | Pulmonary TB | Adults | PCR |
| Loop-LAMP | Detect mutations associated with drug resistance to RIF, INH, and ETO | Pulmonary TB, extra-pulmonary TB | Adults | PCR |
| Lipoarabinomannan (LAM) determination by lateral flow immunochromatography | Detect mycobacterial LAM antigen in urine | Pulmonary TB, extra-pulmonary TB, HIV co-infection | Adults and children | Immunochromatograph |
In 2021, the World Health Organization [46] issued guidelines advocating for tubercular immunological MTB antigen-based skin test, termed TBST. These assays emphasize the ESAT-6 protein and filtrate protein 10 (CFP-10) antigens to trigger the release of IFN-γ from specific T cells. Among the TBSTs evaluated by the WHO are: Cy-Tb (India), which integrates recombinant proteins derived from modified Lactobacillus lactis genes, with a 0.1 mL dose containing 0.05 μg of rd ESAT-6 and 0.05 μg of rCFP-10; Diaskintest®, formulated with a recombinant protein produced by E. coli BL21 (DE3)/pCFP-ESAT, with a 0.1 mL dose delivering 0.2 μg of the recombinant ESAT-6 and CFP10 proteins, endorsed by Russian authorities; and C-TST, employing a recombinant fusion protein of ESAT-6 and CFP-10, expressed in genetically optimized E. coli, with a 0.1 mL dose providing five units (U) of the recombinant Mtb fusion protein, sanctioned by the Chinese government.
Central to the innovation of these tests is their fusion of IGRA specificity with the skin test platform. A study by Hamada et al. [47] juxtaposed the diagnostic capabilities of Diaskintest, ELISPOT, and QFT, revealing sensitivities of 88.7%, 90.6%, and 87.0%, respectively. This underscores the comparable specificity of TBSTs to IGRAs, surpassing that of the TST. Moreover, their specificity matches or even exceeds that of IGRA in pediatric populations and those co-infected with HIV. This was further corroborated by Nikitina et al. [48] who compared Diaskintest and QuantiFERON-TB Gold (QFT) in adults and children suspected of having TB. Their findings indicated an 84% concordance in adults and 90% in children, with heightened diagnostic sensitivity in the latter (73% and 65%, respectively). However, this evidence has yet to undergo systematic review.
In addition to diagnostic sensitivity validation, Starshinova et al. [49] demonstrated that TBSTs are as safe to use as the TST, with over 95% of subjects experiencing only mild injection site reactions, such as swelling, slight pain, and itching. Given its simplicity and cost effectiveness, the TBST emerges as a promising tool to enhance detection and health equity, mitigating the risk of false positives.
Concurrently, there is an ongoing pursuit for novel biomarkers, beyond DNA, that hold the potential to refine and advance more efficient diagnostic methodologies [50]. In this context, there has been increasing interest in the application of untargeted metabolomics, a tool deemed strategic for the identification of a wide range of significant biomarkers, thereby facilitating a better understanding of the host–pathogen interaction [51].
The research conducted by Conde et al. [52] focused on uncovering a set of biomarkers associated with TB diagnosis by conducting a metabolic study of serum through nuclear magnetic resonance. The study group included healthy individuals, TB patients (active TB or LTBI), and patients with pulmonary and extrapulmonary TB. The study indicated that the metabolism of amino acids was the most significantly altered and differentiated, followed by purine metabolism, glyoxylate and dicarboxylate metabolism, and aminoacyl-tRNA biosynthesis. They concluded that inosine, hypoxanthine, mannose, asparagine, aspartate, and glutamate were the six primary metabolites prominently linked to metabolic processes during TB infection. These results can be interpreted considering that the MTB bacterium thrives inside macrophages, confronting an acidic environment with nutrient scarcity.
Metabolomics is also useful for differentiating between various species and TB-XDR strains selectively. In line with this, Huang et al. [53] identified TB-XDR biomarkers using ultra-high-performance liquid chromatography in conjunction with mass spectroscopy (UPLC-Q-TOF-MS), pinpointing four primarily altered metabolites: N1M2P5C, MG3P, CA, and DX. With these, they were able to construct a differential diagnostic model for TB-XDR with an accuracy, sensitivity, and specificity of 0.928, 86.7%, and 86.7%, respectively. Conversely, Chaiyachat et al. [54] discovered metabolic markers that differentiate pre-XDR strains from MTB-XDR using ultra-high-performance liquid chromatography alongside electrospray ionization quadrupole time-of-flight mass spectrometry (UHPLC-ESI-QTOF-MS/MS). They reported that the levels of the metabolites meso-hydroxyheme and itaconic anhydride are responsible for distinguishing between pre-XDR and XDR strains.
Metabolomics can also contribute to the development of methods to differentiate between TB and LTBI. Albors-Vaquer et al. [55] examined the differences in the metabolic profile of patients with active TB and those with LTBI, showing that both groups exhibit a unique serum metabolic profile compared with healthy individuals. They reported that the levels of amino acids such as alanine, lysine, glutamate, glutamine, citrate, and choline decrease in patients with an active infection, as MTB quickly absorbs and metabolizes them as nitrogen sources. In line with this finding, Della Bella et al. [56] developed a new blood analysis called LIOSpot TB that distinguishes TB from LTBI. This test is based on alanine dehydrogenase and has demonstrated that the MTB antigen can stimulate the production of IL-2 in active TB but not in LTBI. Although further validation and comparison with other pulmonary diseases for clinical evaluation are still necessary, these findings represent a significant advancement, highlighting the potential of metabolomics to provide new study targets.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15102409
References
- Vincent, A.T.; Nyongesa, S.; Morneau, I.; Reed, M.B.; Tocheva, E.I.; Veyrier, F.J. The Mycobacterial Cell Envelope: A Relict From the Past or the Result of Recent Evolution? Front. Microbiol. 2018, 9, 2341.
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 5: Management of Tuberculosis in Children and Adolescents. 2022. Available online: https://iris.who.int/handle/10665/352522 (accessed on 20 September 2023).
- Kwok, P.C.L.; Grabarek, A.; Chow, M.Y.T.; Lan, Y.; Li, J.C.W.; Casettari, L.; Mason, A.J.; Lam, J.K.W. Inhalable Spray-Dried Formulation of D-LAK Antimicrobial Peptides Targeting Tuberculosis. Int. J. Pharm. 2015, 491, 367–374.
- Lerner, T.R.; Borel, S.; Gutierrez, M.G. The Innate Immune Response in Human Tuberculosis. Cell Microbiol. 2015, 17, 1277–1285.
- Buccini, D.F.; Cardoso, M.H.; Franco, O.L. Antimicrobial Peptides and Cell-Penetrating Peptides for Treating Intracellular Bacterial Infections. Front. Cell Infect. Microbiol. 2021, 10, 612931.
- Theriault, M.E.; Pisu, D.; Wilburn, K.M.; Lê-Bury, G.; MacNamara, C.W.; Michael Petrassi, H.; Love, M.; Rock, J.M.; VanderVen, B.C.; Russell, D.G. Iron Limitation in M. Tuberculosis Has Broad Impact on Central Carbon Metabolism. Commun. Biol. 2022, 5, 685.
- Rao Muvva, J.; Ahmed, S.; Rekha, R.S.; Kalsum, S.; Groenheit, R.; Schön, T.; Agerberth, B.; Bergman, P.; Brighenti, S. Immunomodulatory Agents Combat Multidrug-Resistant Tuberculosis by Improving Antimicrobial Immunity. J. Infect. Dis. 2021, 224, 332–344.
- Redford, P.S.; Murray, P.J.; O’Garra, A. The Role of IL-10 in Immune Regulation during M. tuberculosis Infection. Mucosal Immunol. 2011, 4, 261–270.
- Hutchins, A.P.; Poulain, S.; Miranda-Saavedra, D. Genome-Wide Analysis of STAT3 Binding in Vivo Predicts Effectors of the Anti-Inflammatory Response in Macrophages. Blood 2012, 119, e110-9.
- Ashhurst, A.S.; Flórido, M.; Lin, L.C.W.; Quan, D.; Armitage, E.; Stifter, S.A.; Stambas, J.; Britton, W.J. CXCR6-Deficiency Improves the Control of Pulmonary Mycobacterium tuberculosis and Influenza Infection Independent of T-Lymphocyte Recruitment to the Lungs. Front. Immunol. 2019, 10, 339.
- World Health Organization. Latent tuberculosis infection: Updated and consolidated guidelines for programmatic management. World Health Organization License: CC BY-NC-SA 3.0 IGO. , 2018. Available online: https://iris.who.int/handle/10665/260233 (accessed on 20 September 2023).
- Centers for Disease Control and Prevention TB Elimination The Difference between Latent TB Infection and TB Disease. Available online: https://www.cdc.gov/tb/ (accessed on 19 February 2023).
- Mohd Shariff, N. Recent Advancement and Future Perspective for the Treatment of Multidrug-Resistant Tuberculosis. In Nanotechnology Based Approaches for Tuberculosis Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 231–250.
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2022.
- Behr, M.A.; Kaufmann, E.; Duffin, J.; Edelstein, P.H.; Ramakrishnan, L. Latent Tuberculosis: Two Centuries of Confusion. Am. J. Respir. Crit. Care Med. 2021, 204, 142–148.
- Houben, R.M.G.J.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-Estimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152.
- Cohen, A.; Mathiasen, V.D.; Schön, T.; Wejse, C. The Global Prevalence of Latent Tuberculosis: A Systematic Review and Meta-Analysis. Eur. Respir. J. 2019, 54, 1900655.
- Cui, D.; Hu, X.; Shi, L.; Wang, D.; Chen, G. Linezolid-Related Adverse Effects in the Treatment of Rifampicin Resistant Tuberculosis: A Retrospective Study. J. Chemother. 2022, 35, 404–410.
- Abha Majumdar Should Latent Tuberculosis Be Treated? Available online: https://www.drabhamajumdar.com/tuberculosis.html (accessed on 9 April 2023).
- Centers for Disease Control and Prevention Tuberculosis (TB). Available online: https://www.cdc.gov/tb/esp/statistics/default.htm (accessed on 11 April 2023).
- Gupta, S.K.; Angara, R.K.; Yousuf, S.; Reddy, C.G.; Ranjan, A. Ectopic Expression of Rv0023 Mediates Isoniazid/Ethionamide Tolerance via Altering NADH/NAD+ Levels in Mycobacterium smegmatis. Front. Microbiol. 2020, 11, 3.
- Basera, T.J.; Ncayiyana, J.; Engel, M.E. Prevalence and Risk Factors of Latent Tuberculosis Infection in Africa: A Systematic Review and Meta-Analysis Protocol. BMJ Open 2017, 7, e012636.
- Guthmann, J.-P.; Haas, W. Tuberculosis in the European Union/European Economic Area: Much Progress, Still Many Challenges. Eurosurveillance 2019, 24, 1900174.
- Knight, G.M.; McQuaid, C.F.; Dodd, P.J.; Houben, R.M.G.J. Global Burden of Latent Multidrug-Resistant Tuberculosis: Trends and Estimates Based on Mathematical Modelling. Lancet Infect. Dis. 2019, 19, 903–912.
- Theran León, J.S.; Esteban Badillo, L.Y.; Villalobos, M.A.; Dulcey, L.A. Coinfección de Tuberculosis y COVID-19 Asociado a Tromboembolismo Pulmonar: Presentación de Un Caso. Atención Primaria Práctica 2022, 4, 100129.
- Abdulkareem, F.N.; Merza, M.A.; Salih, A.M. First Insight into Latent Tuberculosis Infection among Household Contacts of Tuberculosis Patients in Duhok, Iraqi Kurdistan: Using Tuberculin Skin Test and QuantiFERON-TB Gold Plus Test. Int. J. Infect. Dis. 2020, 96, 97–104.
- Kruczak, K.; Mastalerz, L.; Sładek, K. Interferon-Gamma Release Assays and Tuberculin Skin Testing for Diagnosing Latent Mycobacterium tuberculosis Infection in at-Risk Groups in Poland. Int. J. Mycobacteriol. 2016, 5, 27–33.
- Chapman, H.J.; Lauzardo, M. Advances in Diagnosis and Treatment of Latent Tuberculosis Infection. J. Am. Board Fam. Med. 2014, 27, 704–712.
- Iannone, F.; Cantini, F.; Lapadula, G. Diagnosis of Latent Tuberculosis and Prevention of Reactivation in Rheumatic Patients Receiving Biologic Therapy: International Recommendations. J. Rheumatol. Suppl. 2014, 91, 41–46.
- Kussen, G.M.B.; Dalla-Costa, L.M.; Rossoni, A.; Raboni, S.M. Interferon-Gamma Release Assay versus Tuberculin Skin Test for Latent Tuberculosis Infection among HIV Patients in Brazil. Braz. J. Infect. Dis. 2016, 20, 69–75.
- Turetz, M.L.; Ma, K.C. Diagnosis and Management of Latent Tuberculosis. Curr. Opin. Infect. Dis. 2016, 29, 205–211.
- Ahmad, S. New Approaches in the Diagnosis and Treatment of Latent Tuberculosis Infection. Respir. Res. 2010, 11, 169.
- Schluger, N. Advances in the Diagnosis of Latent Tuberculosis Infection. Semin. Respir. Crit. Care Med. 2013, 34, 060–066.
- Park, C.H.; Park, J.H.; Jung, Y.S. Impact of Immunosuppressive Therapy on the Performance of Latent Tuberculosis Screening Tests in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 507.
- Kang, Y.-J.; Park, H.; Park, S.-B.; Kim, J.; Lee, J.; Kim, J.; Park, S.; Lee, Y.S.; Kim, S. Combined Analysis of Whole Blood Interferon Gamma Release Assay and Complete Blood Count Analysis for Rapid Discrimination of Active Tuberculosis and Latent Tuberculosis Infection. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 24, 100253.
- Pai, M.; Denkinger, C.M.; Kik, S.V.; Rangaka, M.X.; Zwerling, A.; Oxlade, O.; Metcalfe, J.Z.; Cattamanchi, A.; Dowdy, D.W.; Dheda, K.; et al. Gamma Interferon Release Assays for Detection of Mycobacterium tuberculosis Infection. Clin. Microbiol. Rev. 2014, 27, 3–20.
- Petruccioli, E.; Chiacchio, T.; Pepponi, I.; Vanini, V.; Urso, R.; Cuzzi, G.; Barcellini, L.; Cirillo, D.M.; Palmieri, F.; Ippolito, G.; et al. First Characterization of the CD4 and CD8 T-Cell Responses to QuantiFERON-TB Plus. J. Infect. 2016, 73, 588–597.
- Petruccioli, E.; Vanini, V.; Chiacchio, T.; Cuzzi, G.; Cirillo, D.M.; Palmieri, F.; Ippolito, G.; Goletti, D. Analytical Evaluation of QuantiFERON- Plus and QuantiFERON- Gold In-Tube Assays in Subjects with or without Tuberculosis. Tuberculosis 2017, 106, 38–43.
- Lewinsohn, D.A.; Heinzel, A.S.; Gardner, J.M.; Zhu, L.; Alderson, M.R.; Lewinsohn, D.M. Mycobacterium tuberculosis–Specific CD8+ T Cells Preferentially Recognize Heavily Infected Cells. Am. J. Respir. Crit. Care Med. 2003, 168, 1346–1352.
- Petruccioli, E.; Chiacchio, T.; Vanini, V.; Cuzzi, G.; Codecasa, L.R.; Ferrarese, M.; Schininà, V.; Palmieri, F.; Ippolito, G.; Goletti, D. Effect of Therapy on Quantiferon-Plus Response in Patients with Active and Latent Tuberculosis Infection. Sci. Rep. 2018, 8, 15626.
- Du, F.; Xie, L.; Zhang, Y.; Gao, F.; Zhang, H.; Chen, W.; Sun, B.; Sha, W.; Fang, Y.; Jia, H.; et al. Prospective Comparison of QFT-GIT and T-SPOT.TB Assays for Diagnosis of Active Tuberculosis. Sci. Rep. 2018, 8, 5882.
- Yang, Q.; Xu, Q.; Chen, Q.; Li, J.; Zhang, M.; Cai, Y.; Liu, H.; Zhou, Y.; Deng, G.; Deng, Q.; et al. Discriminating Active Tuberculosis from Latent Tuberculosis Infection by Flow Cytometric Measurement of CD161-Expressing T Cells. Sci. Rep. 2015, 5, 17918.
- Jyotirmoy Das; Nina Idh; Isabelle Pehrson; Jakob Paues; Maria Lerm A DNA Methylome Biosignature in Alveolar Macrophages from TB-Exposed Individuals Predicts Exposure to Mycobacteria. MedRxiv 2021.
- Karlsson, L.; Das, J.; Nilsson, M.; Tyrén, A.; Pehrson, I.; Idh, N.; Sayyab, S.; Paues, J.; Ugarte-Gil, C.; Méndez-Aranda, M.; et al. A Differential DNA Methylome Signature of Pulmonary Immune Cells from Individuals Converting to Latent Tuberculosis Infection. Sci. Rep. 2021, 11, 19418.
- Liu, W.; Zou, D.; He, X.; Ao, D.; Su, Y.; Yang, Z.; Huang, S.; Zhao, Q.; Tang, Y.; Ma, W.; et al. Development and Application of a Rapid Mycobacterium tuberculosis Detection Technique Using Polymerase Spiral Reaction. Sci. Rep. 2018, 8, 3003.
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 3: Diagnosis: Rapid Diagnostics for Tuberculosis Detection. 2020. Available online: https://iris.who.int/handle/10665/332862 (accessed on 20 September 2023).
- Hamada, Y.; Kontsevaya, I.; Surkova, E.; Wang, T.T.; Wan-Hsin, L.; Matveev, A.; Ziganshina, L.E.; Denkinger, C.M.; Korobitsyn, A.; Ismail, N.; et al. A Systematic Review on the Safety of Mycobacterium Tuberculosis –Specific Antigen–Based Skin Tests for Tuberculosis Infection Compared with Tuberculin Skin Tests. Open Forum Infect. Dis. 2023, 10, 228.
- Nikitina, I.Y.; Karpina, N.L.; Kasimceva, O.V.; Gergert, V.Y.; Ergeshov, A.; Lyadova, I.V. Comparative Performance of QuantiFERON-TB Gold versus Skin Test with Tuberculosis Recombinant Allergen (Diaskintest) among Patients with Suspected Pulmonary Tuberculosis in Russia. Int. J. Infect. Dis. 2019, 86, 18–24.
- Starshinova, A.; Zhuravlev, V.; Dovgaluk, I.; Panteleev, A.; Manina, V.; Zinchenko, U.; Istomina, E.; Pavlova, M.; Yablonskiy, P. A Comparison of Intradermal Test with Recombinant Tuberculosis Allergen (Diaskintest) with Other Immunologic Tests in the Diagnosis of Tuberculosis Infection. Int. J. Mycobacteriol. 2018, 7, 32.
- Acharya, B.; Acharya, A.; Gautam, S.; Ghimire, S.P.; Mishra, G.; Parajuli, N.; Sapkota, B. Advances in Diagnosis of Tuberculosis: An Update into Molecular Diagnosis of Mycobacterium tuberculosis. Mol. Biol. Rep. 2020, 47, 4065–4075.
- Preez, I.D.; Luies, L.; Loots, D.T. Metabolomics Biomarkers for Tuberculosis Diagnostics: Current Status and Future Objectives. Biomark. Med. 2017, 11, 179–194.
- Conde, R.; Laires, R.; Gonçalves, L.G.; Rizvi, A.; Barroso, C.; Villar, M.; Macedo, R.; Simões, M.J.; Gaddam, S.; Lamosa, P.; et al. Discovery of Serum Biomarkers for Diagnosis of Tuberculosis by NMR Metabolomics Including Cross-Validation with a Second Cohort. Biomed. J. 2022, 45, 654–664.
- Huang, H.; Han, Y.-S.; Chen, J.; Shi, L.-Y.; Wei, L.-L.; Jiang, T.-T.; Yi, W.-J.; Yu, Y.; Li, Z.-B.; Li, J.-C. The Novel Potential Biomarkers for Multidrug-Resistance Tuberculosis Using UPLC-Q-TOF-MS. Exp. Biol. Med. 2020, 245, 501–511.
- Chaiyachat, P.; Kaewseekhao, B.; Chaiprasert, A.; Kamolwat, P.; Nonghanphithak, D.; Phetcharaburanin, J.; Sirichoat, A.; Ong, R.T.-H.; Faksri, K. Metabolomic Analysis of Mycobacterium tuberculosis Reveals Metabolic Profiles for Identification of Drug-Resistant Tuberculosis. Sci. Rep. 2023, 13, 8655.
- Albors-Vaquer, A.; Rizvi, A.; Matzapetakis, M.; Lamosa, P.; Coelho, A.V.; Patel, A.B.; Mande, S.C.; Gaddam, S.; Pineda-Lucena, A.; Banerjee, S.; et al. Active and Prospective Latent Tuberculosis Are Associated with Different Metabolomic Profiles: Clinical Potential for the Identification of Rapid and Non-Invasive Biomarkers. Emerg. Microbes Infect. 2020, 9, 1131–1139.
- Della Bella, C.; Spinicci, M.; Grassi, A.; Bartalesi, F.; Benagiano, M.; Truthmann, K.; Tapinassi, S.; Troilo, A.; D’Elios, S.; Alnwaisri, H.; et al. Novel, M. tuberculosis Specific IL-2 ELISpot Assay Discriminates Adult Patients with Active or Latent Tuberculosis. PLoS ONE 2018, 13, e0197825.
This entry is offline, you can click here to edit this entry!
