Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Developmental Biology
Conventional spinach breeding is limited by the very complex sex determination. However, these limitations could be circumvented in synergy with a biotechnological approach. Accordingly, tissue culture techniques allow rapid and efficient clonal propagation of selected valuable genotypes, and somatic embryogenesis has been recognized as a superior process for clonal propagation because somatic embryos resemble zygotic embryos and therefore can spontaneously develop into complete plants.
- Amaranthaceae
- gene expression
- gibberellins
- somatic embryogenesis
- Spinacia oleracea L.
- tissue culture
1. Introduction
Somatic embryogenesis in spinach was achieved in parallel in two laboratories in 1993, one in France [96] and the other in the authors’ laboratory in Serbia [97]. In the following decades, numerous reports have been published on this subject, and the mechanism of SE induction and the importance of the factors affecting this process have become better understood. However, the established protocols are still genotype-dependent and require further improvement, as a robust and generally applicable protocol for efficient and reliable SE induction is needed for further application in biotechnological crop improvement.
In all studies, somatic embryogenesis was induced indirectly via an intermediate callus phase [69,70,84,96,97,98,99,100,101,102]. Rarely was the direct formation of SEs from the leaves of SE-derived plants observed [98]. In some regeneration systems, somatic embryogenesis and de novo shoot organogenesis proceeded in parallel [84,96,97,98,99,101], while in the majority of studies only SEs were observed [69,70,71,100,102,103,104,105]. In some cases, shoot organogenesis preceded the formation of SEs [96,97,98,99], while Nguyen et al. [84] were able to alternately induce SEs or shoot buds from the same explant type by controlling the combination and concentration of plant growth regulators (PGRs). Interestingly, the same regeneration procedure using root explants resulted in the regeneration of shoot buds in some cultivars [83], while in others only SEs were formed [71,102,105]. It is also worth noting that explants of the same cultivar produced exclusively SEs in one procedure [71,102,105] and both SEs and shoot buds in another [97,98,99].
2. Factors Affecting Somatic Embryogenesis
Numerous factors significantly influence somatic embryogenesis in spinach. The most important effect on this process is the genotype, explant type, media composition (particularly the PGR combination, concentration, and treatment duration), and environmental factors such as light conditions and temperature. The effects of these factors on the initiation of SEs are presented on the following pages and summarized in Table 1.
To facilitate the understanding the text, the following terminology is used: “embryogenic capacity” is used as a general descriptive measure of the ability of explants to regenerate SEs, whereas “embryogenic response” indicates that explants are able to regenerate SEs in response to SE induction treatment. “Regeneration frequency” (defined as the proportion of explants regenerating SEs out of the total number of explants subjected to SE induction treatment) and “mean SE number” per explant are used to quantitatively describe SE regeneration.
2.1. Genotype
Genotype has been recognized as one of the most important factors that strongly influences not only somatic embryogenesis [69,70,71,102] but also callus induction [68] and the regeneration of shoot buds from calli or protoplasts [68,90]. The frequency of SE regeneration from seedling root segments varied widely among the cultivars tested [69,101]. A single study tested the frequency of SE regeneration in eight spinach cultivars: Jiromaru, Nihon, Hoyo, Minsterland, Virofly, King of Denmark, Nobel, and Ujo [69]. The frequency of SE regeneration varied from 17.1% (in the cultivar Ujo) to 78% (in the cultivar Jiromaru) [69]. However, results obtained by the same research group in different experiments under the same experimental conditions showed high variability in the embryogenic response of seedling root segments within a highly responsive cultivar (Jiromaru), ranging from 36% to 78% [69,100,104]. Overall, these results suggest large differences in individual (seedling) response between and within seed lots. Indeed, a large variation in the embryogenic response of root sections isolated from 30 randomly selected seedlings of the cultivar Nippon was later found following the same regeneration protocol in the same laboratory [70]. Of 30 seedlings tested, explants from 10 seedlings had low, 13 had medium, and seven had high embryogenic capacity, with 20%, 20–70%, and 80% embryogenic explants per seedling, respectively [70].
A rather similar embryogenic response was observed for root tips of the cultivar Matador, previously considered extremely recalcitrant to in vitro regeneration [72]. Out of 30 randomly tested seedlings at the same developmental stage (4–5 leaves), root apices of 26 seedlings responded with SE regeneration frequencies ranging from 0.3% to 100%, while roots of four seedlings did not respond at all [102]. The mean SE number per explant in the responsive lines ranged from 0.001 to 9.96, and 227–347 SEs were obtained from 30 root explants of each of the four most responsive lines over 12 weeks [102]. The obtained SEs developed into plants, flowered, and set seed in vitro, so the embryogenic response of the resulting 69 seedlings was tested [102]. The progeny of the poorly responding parental lines always responded in a similar manner, with SE regeneration frequencies below 12% and up to 0.47 SEs per explant, while the lines with moderate embryogenic capacity (with SE regeneration frequencies of 20–70%) produced progeny with highly variable SE regeneration frequencies (0.86–98.5%) and mean SE numbers per explant (0.001–10.41). However, one line that showed moderate embryogenic response (35.3% and 8.74 SEs per explant, 115 SEs from 30 explants) resulted in progeny with high embryogenic capacity after 3–4 cycles of self-fertilization [102]. The root apices of these lines regenerated SEs at a frequency of 96.4–100%, with 40.6–68.3 SEs per explant, and produced 1547–2181 SEs from 30 explants over 12 weeks [102]. This was 13.4–19.0 times more than the mother plant and 4.5–6.3 times more than the explants of the best performing seedling randomly selected from the seed lot tested in the same study [102]. In fact, in later experiments, we tested the embryogenic capacity of almost one thousand randomly selected seedlings in different studies, and none of them showed even close embryogenic capacity compared to the four elite lines. Moreover, the above-mentioned highly responsive lines regenerated SEs much faster, starting from the fourth week of culture, while lines with low embryogenic capacity regenerated SEs most frequently only after 6–8 weeks of culture initiation [102]. Roots isolated from SE-derived plants behaved similarly to the roots of the corresponding seedlings, suggesting the temporal stability of this trait within the line [102]. The four elite lines obtained in this study were maintained through cycles of SE-induction from the roots of SE-derived plants, which retained a high embryogenic capacity for 5–7 years. In agreement with this, Ishizaki et al. [106] maintained root cultures of highly responsive lines on PGR-free medium and found that their embryogenic capacity was high and stable for at least 12 months.
Deeper insights into the intrinsic variability of the embryogenic capacity in spinach were obtained by studying embryogenic capacity at both population and individual (single seedling) levels in the cultivar Matador [71]. Large differences were found among seeds of the Matador cultivar obtained from nine European seed companies from Slovenia (Sl), Poland (P), Serbia (Sr), England (E), Germany (G), Lithuania (L), Ukraine (U), Russia (R), and Italy (I). The frequency of seedlings with embryogenic capacity (i.e., seedlings whose root apices regenerated at least one SE) was highest in population Sl (100%), followed by P (98%), Sr and E (88% each), G (60%), L (58%), U (34%), R, and I (0% each) during a 12-week cultivation period [71]. The mean SE number per root explant, calculated at the population level, was also highest in the Sl population (14.4), followed by the P, Sr, and E (2.6–4.1), G, L, and U (0.3–0.6), and R and I (0 each) populations. The speed of regeneration of SEs from root explants followed the same order as the frequency and mean SE number, from the fastest to the slowest: Sl, P, Sr, G, E, L, U, R, and I [71]. The first SEs were observed on Sl explants in the fourth week of culture initiation in 10.3% of Sl seedlings, and a 100% regeneration response was achieved in the seventh week of culture initiation.
At the seedling level, the Sl population was found to be composed of highly responsive seedlings, as 82% of Sl seedlings had 80–100% regenerating explants, whereas in the intermediate responsive populations (Sr, P, and E), 21–28% of seedlings were non-responsive and 40% of seedlings had 20–60% regenerating explants [71]. As expected, in the least responsive populations (G, L, and U), explants from 52–85% of seedlings were non-responsive, and explants from only up to 1.3% of seedlings regenerated SEs at a frequency greater than 60%. Accordingly, explants from Sl seedlings regenerated the highest number of SEs: 27.6% of seedlings regenerated more than 20 SEs per explant, 43.5% regenerated 10–20 SEs, and only 19.6% regenerated 1–10 SEs per explant [71]. In contrast, 73.8%, 59.7%, and 36.1% of P, E, and G seedlings, respectively, regenerated only 1–10 SEs per explant.
The observed large differences in the embryogenic capacity of seedlings may be due to genotypic effects, the physiological age of the seed at harvest, and postharvest storage conditions [107]. Because the seeds used in the above study were produced under different climatic conditions, the physiological age of the seeds at harvest is unknown, and the seeds were stored in different storage locations until shipment. Selected seed populations (Sl, Sr, and U) of the same seed lots used in the study by Belić et al. [71] were sown and grown under the same environmental conditions, and the embryogenic capacities of the root apices of the obtained seedlings were compared. Seeds from each seed population were sown in a separate planter, and each planter was placed in an isolated cage to prevent cross-pollination between plants from the different seed populations, as suggested by Morelock and Correll [2]. The root apices of the obtained seedlings showed a similar embryogenic response as that in the study by Belić et al. [71]: Sl seedling explants had the highest embryogenic capacity, Sr medium, and U very low (Figure 1) (Zdravković-Korać et al., unpublished results), indicating a strong influence of genotype on the embryogenic response [71]. Therefore, due to the significant variation between lots, it is strongly recommended to test the plant material (seed lot) prior to any research on somatic embryogenesis [71].
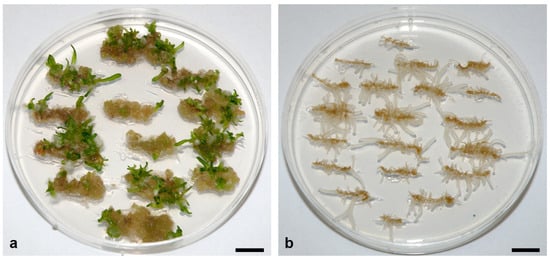
Figure 1. Regeneration of somatic embryos (SEs) from root apices of randomly selected seedlings from (a) Slovenian (Sl) and (b) Ukrainian (U) populations of the spinach cultivar Matador. Root apices were cultivated for eight weeks on MS medium supplemented with 20 μM NAA + 5 μM GA3 under a long-day photoperiod (16 h of light) and a photosynthetic photon flux density of 100 μmol m−2 s−1. Regeneration of the SEs from Sl explants began from the fifth week of culture. Scale bar: 1 cm. Unpublished from the authors’ laboratory.
2.2. Explant Type
Numerous explant types have been used to induce somatic embryogenesis in spinach (Table 1). In most studies, seedling organs have been used as explants, not only because young organs generally respond more efficiently to induction treatments than older ones, but also because seeds are more robust than other plant organs and allow the use of more stringent sterilization procedures to successfully eliminate microorganisms [98,101].
Comparative studies have examined the frequency of SE induction from hypocotyls, cotyledons, and roots [69,101]. Seedlings were typically 8–10 days old, and sections were 5–8 mm long [69] or 400–500 μm thick transverse thin cell layer (TCL) sections [101] (Table 1). In addition, 2–3 mm long hypocotyl segments [96], the hypocotyl and middle part of cotyledons (5–10 mm), and leaf discs (5 mm) isolated from 1–2 month-old plants were also examined [69,98,99].
Of all explants, root sections responded most efficiently to the SE induction treatments [69,100,101,104]. In the cultivar Jiromaru, 75% of the root sections formed embryogenic callus, followed by the hypocotyl segments and basal cotyledon segments (28% and 16%, respectively), while the distal cotyledon segments formed hardly any calli and showed no embryogenic capacity [69]. In the cultivar Carpo, only root sections responded with a frequency of up to 25% in a series of treatments [101]. Interestingly, in the same study, in cultivar RZ1, only a few (0.79%) hypocotyl TCLs responded, while root and cotyledon TCLs did not [101]. Similarly, in the cultivars Matador and Virofly, hypocotyl-derived calli were more frequently embryogenic (3.7% and 5.7%, respectively) than cotyledon-derived calli (1.07% and 0%, respectively) [98]. However, in the cultivar Matador, leaf discs derived from 2-month-old greenhouse plants were the most responsive explants (83%) [98], whereas leaf segments isolated from 30-day-old in vitro-cultured plants of the cultivar Jiromaru showed no embryogenic capacity [69].
Because root segments had the highest embryogenic capacity in most studies, they were the preferred explants in subsequent studies of somatic embryogenesis in spinach [70,71,84,102,105,108,109,110]. Moreover, some studies found that the regeneration response differed along the roots—the apical and middle fragments of the roots responded more strongly than the basal ones [83], which is why some authors used only the root apices [71,102,105,108,109,110]. However, Ishizaki et al. [70] used root sections (5 mm) from previously established root cultures grown on PGR-free medium, and Nguyen et al. [84] used 5–10 mm long main and lateral root fragments without root tips to induce somatic embryogenesis.
As mentioned above, organs from young seedlings 1–2 weeks old were most commonly used for culture initiation. However, the effect of donor plant age on the SE regeneration capacity has never been studied in spinach, although it has been shown that cotyledons from five-day-old seedlings showed the best de novo shoot regeneration response compared to younger and especially older cotyledons that lacked the ability to regenerate shoots [81].
Somatic embryogenesis was also induced from protoplasts obtained from the cotyledons, roots, and hypocotyls of 10-day-old seedlings and from the leaves of 30-day-old plants of the cultivar Jiromaru [91].
Table 1. Summary of literature data on the induction of somatic embryogenesis from different types of explants isolated from different sources in spinach cultivars. The content of plant growth regulators in the media used for the induction of embryogenic callus and somatic embryo regeneration is shown in the table.
| Cultivar | Explant Source | Explant Types Tested | Callus Induction | SE * Regeneration | Reference |
|---|---|---|---|---|---|
| Carpo | 1-week-old seedlings | Hypocotyl segments (2–3 mm) | MS + 85.7 μM IAA + 100 μM GA3 + 2% sucrose | MS + 11.4 μM IAA + 10 μM GA3 + 2% sucrose | [96] |
| Carpo, RZ1 | 8-day-old seedlings | Cotyledon, hypocotyl, root (TCL: 400–500 μm) | MS + 100 μM NAA + 1 μM BA + 10 μM GA3 + 5% sucrose | MS, PGR-free | [101] |
| Gyeowoonae | 2-week-old seedlings | Root segments (5–10 mm) | 1/2 MS + 10 μM NAA + 0.3 μM GA3 + 2% sucrose | 10μM NAA + 0.1 μM GA3 + 2% sucrose | [84] |
| Jiromaru, Nihon, Hoyo, Minsterland, Viroflay, King of Denmark, Nobel, Ujo | 10-day-old seedlings 30-day-old plants |
Cotyledon, hypocotyl, root segments (5 mm each), leaf fragments (5 mm2) | MS/2 + 10 μM NAA + 0.1 μM GA3 + 1% sucrose | MS, PGR-free + 2% sucrose | [69] |
| Jiromaru | 10-day-old seedlings | Root segments (8 mm) | MS/2 + 30 μM NAA + 100 μM GA3 + 1% sucrose | MS, PGR-free + 2% sucrose | [100] |
| Jiromaru | 10-day-old seedlings | Root segments (8 mm) | MS/2 + 30 μM NAA + 100 μM GA3 + 29 mM fructose | MS, PGR-free + 59 mM sucrose | [104] |
| Matador | 2-month-old plants | Leaf discs (5 mm) | MS + 4.4 μM 2,4-D + 4.6 μM Kin + 2% sucrose | MS + 4.6 μM Kin + 2% sucrose | [97,99] |
| Matador, Virofly | 2-month-old plants, 10-day-old seedlings |
Leaf discs (5 mm), hypocotyl segments (5–10 mm), middle part of cotyledons |
MS + 4.4 μM 2,4-D + 4.6 μM Kin + 2% sucrose | MS + 4.6 μM Kin + 2% sucrose | [98] |
| Nippon | in vitro root culture | Root segments (5 mm) | 1/2 MS + 10 μM NAA + 0.1 μM GA3 + 5.4 g/L fructose | 1/2 MS, PGR-free + 2% sucrose | [70] |
* SE—somatic embryo; 2,4-D—2,4-dichlorophenoxyacetic acid; Kin—kinetin; IAA—indole-3-acetic acid; GA3—gibberellic acid; NAA—α-naphthaleneacetic acid; BA—benzyladenine.
2.3. Media Composition—Mineral Elements
In most studies, a full or half-strength MS mineral solution was used for embryogenic callus induction in spinach (Table 1) [96,97,98,99]. However, Nitsch’s and 1/2 MS solutions were found to induce the highest frequency of embryogenic response in root sections of the Jiromaru cultivar (51.1% and 37.8%, respectively) among the six mineral solutions tested: White’s [111], MS [112], half-strength MS (1/2 MS), B5 [113], Nitsch’s [114], and SH [115], whereas explants cultured on medium supplemented with White’s mineral solution showed no embryogenic response [69]. The ratio of nitrate and ammonium in a mineral solution also had a significant effect on embryogenic response, and the highest frequency of embryogenic explants (62.2%) was obtained in the explants cultured at a nitrate:ammonium ratio of 2:1 [69]. The total nitrogen concentration (nitrate + ammonium) was optimal at a nitrate:ammonium ratio of 2:1 at 10–30 mM [69], which is why these authors used 1/2 MS mineral solution for embryogenic callus induction in later studies [69,70]. However, Leguillon et al. [101] compared the effects of MS and MW mineral solution, the latter containing White’s macroelements, Nitch’s microelements, and MS vitamins, and obtained better results with MW, although the regeneration response was generally low. For SE regeneration, the MS mineral solution was used in all studies (Table 1).
2.4. Plant Growth Regulators
The most common approach to induce somatic embryogenesis in plants is to administer auxin for a short period of time and then withdraw it to allow SEs to develop [116]. However, this approach failed in spinach. Komai et al. [100] tested several of the most commonly used auxins: indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), NAA, and 2,4-dichlorophenoxyacetic acid (2,4-D) at a wide range of concentrations (0.1–100 μM) and found that none of them was sufficient to induce somatic embryogenesis from root sections of the cultivar Jiromaru. Moreover, combinations of these auxins with a number of cytokinins: trans-zeatin [6-(4-hydroxy-3-methylbut-trans-2-enylamino) purine] (ZEA), 2-isopentenyl-adenine (2-iP), N6-furfuryladenine (Kinetin, Kin), N6-benzyladenine (BA), and N-(2-chloro-4-pyridyl)-N′-phenylurea (CPPU) at 0.01–10 μM, did not yield satisfactory results, as only several combinations of NAA or 2,4-D with ZEA or 2-iP elicited embryogenic callus formation and SE regeneration, but with very low frequency (up to 16%). Other research has also tried numerous combinations of Kin with 2,4-D, IAA, or NAA and failed to induce a response in several explant types of the Carpo and RZ1 cultivars [96,101].
Calli derived from leaf discs and hypocotyls of the cultivar Matador on medium supplemented with 4.4 μM 2,4-D + 4.6 μM Kin for 8 weeks and then transferred to medium supplemented with 4.6 μM Kin, however, gradually transformed into a new callus type—a deep red, compact callus within the next 8–12 weeks [97,98,99]. The greenish and red callus types survived side by side in the same cultures, but SEs were observed only on the red parts of the calli. Thus, SEs developed only secondarily on organogenic calli grown on Kin-supplemented medium, in 33.3% of callus clones derived from leaves and 36.4% of callus clones derived from hypocotyls. Calli continuously cultured on callus induction medium containing 4.4 μM 2,4-D + 4.6 μM Kin were organogenic and retained their bud regeneration capacity for up to 10 months, whereas those cultured on Kin-supplemented medium were embryogenic and retained their embryogenic capacity for more than 7 years [98,99]. Repeated rounds of cultivation of red callus on callus induction and regeneration media always followed the same pattern: the formation of green undifferentiated callus on callus induction medium (4.4 μM 2,4-D + 4.6 μM Kin), which gradually became organogenic, and the subsequent formation of red callus, which regenerated SEs after transfer to medium supplemented with 4.6 μM Kin [98].
Moreover, an inverse relationship between callus growth and SE induction has been observed [98,99]. Similarly, an inverse correlation between callus growth and shoot bud regeneration has been observed in several spinach cultivars [74,85]. A good example of this observation is the differential effect of two structurally similar cytokinins, BA and Kin, on these processes. Compared with Kin, BA significantly promoted callus growth but suppressed SE differentiation and vice versa [98]. Consistent with this, 4 and 10 μM abscisic acid (ABA) in combination with BA or Kin decreased callus weight to 20–50% of controls but contributed to a further increase in SE number when combined with Kin. The highest SE number per 1 g of callus (678 SEs/g) was achieved with 4 μM ABA + 5 μM Kin and was almost twice as high as with 5 μM Kin alone (325 SEs/g) [99]. The effect of ABA suggests that the enhancement of somatic embryogenesis in its presence was probably a stress response of the cells, as the cells were prevented from dividing [117]. Conversely, the inhibition of somatic embryogenesis was observed when red embryogenic callus was cultured on BA- or Kin-supplemented media in combination with 0.3, 1, 3 or 10 μM GA3, whereas GA3 significantly promoted callus growth, especially in combination with BA [98,99].
However, in other regeneration systems, GA3 has been found to be essential for SE induction (Table 1) [96,100,101]. Xiao and Branchard [96] applied IAA at high concentrations (85.7 or 48.6 μM) in combination with GA3 (10 or 100 μM) to induce somatic embryogenesis from hypocotyl discs (2–3 mm) of one-week-old seedlings of the cultivar Carpo (Table 1). Embryo-like structures were observed on calli after only three weeks of cultivation on media supplemented with 85.7 μM IAA + 10 or 100 μM GA3, but SEs were obtained from explants cultured on all of the above IAA/GA3 combinations in callus induction medium after an additional four-week period of cultivation. The highest frequency of embryo-forming calli was obtained in explants cultured on medium supplemented with 85.7 μM IAA + 100 μM GA3 for seven weeks and then subcultured on medium containing 11.4 μM IAA + 10 μM GA3 for four weeks (Table 1). In addition, subculturing the calli in liquid medium supplemented with 2.86 μM IAA + 10 μM GA3 was beneficial for the release of SEs from the calli, but with a higher frequency (10%) of SE malformations [96].
Consistent with this, auxins, which were not sufficient to induce embryogenic callus formation from root segments of the cultivar Jiromaru, either alone or in combination with cytokinins, were able to induce somatic embryogenesis when combined with GA3 [100], confirming the requirement of GA3 for SE induction in spinach postulated by Xiao and Branchard [96]. However, GA3 alone was not sufficient to induce cell proliferation or to initiate SEs (Figure 2a). The optimal amount of auxins and GA3 varied among cultivars. In contrast to the cultivar Carpo, high concentrations of IAA (100 μM) and GA3 (1–100 μM) were not effective for SE induction from root explants of the cultivar Jiromaru, with only 4–12% of explants responding with 9.0 SEs per explant [100]. In combination with GA3, IBA and 2,4-D caused a rather low frequency (up to 20%) of embryogenic callus formation, while 10 and 30 μM NAA proved to be much more effective, causing embryogenic callus formation in 16–72% of explants, with 30 μM NAA + 100 μM GA3 being the best combination (Table 1) [100].

Figure 2. Proliferation and SE regeneration from spinach root apices cultured for eight weeks on medium supplemented with (a) 5 μM GA3, where root apices only elongated but did not proliferate or regenerate SEs; (b,d) 20 μM NAA, where abundant proliferation occurred, but SE formation was extremely rare; (c,e) 20 μM NAA + 5 μM GA3, where explants proliferated and regenerated SEs. Three repetitions with 15 explants in each repetition were used per treatment (n = 45). Scale bars: 1 cm. Unpublished from the authors’ laboratory.
Later, the same group of authors demonstrated that GA3 is not required for the induction of SEs from the hairy roots of four spinach cultivars [118]. This phenomenon has not been clearly elucidated, although hairy roots are known to exhibit altered hormone homeostasis [119] and sensitivity to growth regulators [120,121], but no study has addressed this issue.
In another study, 20 μM NAA + 5 μM GA3 proved to be the most effective PGR combination for inducing shoot buds from root sections of the cultivars RS no. E and Longstanding Round [83]. However, the same treatment induced somatic embryogenesis from root sections of the cultivar Matador, while shoot regeneration was never observed under these conditions [71,102,105,108,109].
Ethylene also plays an important role in the induction of somatic embryogenesis from spinach roots, in a complex manner—it is promotive for the induction of embryogenic callus but inhibitory for SE differentiation [122]. Ethephon (1–100 μM), an ethylene-releasing compound, increased the frequency of embryogenic callus formation, but only in combination with 0.1 μM GA3. Conversely, 2-aminoethoxyvinylglycine (AVG), an inhibitor of ethylene biosynthesis, and silver ions, which inhibit ethylene signaling, suppressed embryogenic callus formation at 10 μM and 1 μM, respectively, but significantly increased callus proliferation. The inhibitory effect of 1 μM AVG was abolished by the application of 10 μM ethephon. However, when ethephon was applied after the callus induction phase, i.e., added to the PGR-free medium through the SE proliferation phase, it strongly inhibited SE differentiation. Conversely, silver ions promoted SE differentiation at 1–100 μM, with the effect being strongest at 10 μM [122].
Not only PGR concentration but also treatment duration affected embryogenic efficiency. Komai et al. [69] applied callus induction treatment for only 4 weeks and then subcultured the explants on PGR-free medium. However, many reports showed that longer periods of callus induction were more effective [84,96,98,99]. In the cultivar Gyeowoonae, a 6-week callus induction was much more effective than shorter ones (2 and 4 weeks) for the acquisition of embryogenic competence [84].
2.5. Other Media Components
Carbohydrates are also a mandatory component of the medium. For the induction of embryogenic callus, 2% sucrose has been used most frequently (Table 1) [96,97,98,99]. However, Komai et al. [104] showed that sucrose is not the best choice for efficient SE induction from root explants of the Jiromaru cultivar. The highest frequency of embryogenic callus formation was obtained with fructose (72.5%), raffinose (64.4%), and maltose (52.0%), while glucose (38.6%) and sucrose (36.0%) showed a more modest effect, and mannose and sorbose completely inhibited cell proliferation and callus formation, so these sugars, together with galactose and lactose, were not suitable for cell proliferation. The effect of these hexoses and oligosaccharides was tested at 29, 87, and 145 mM, and all sugars showed the best effect at the lowest concentration of 29 mM [104]. Moreover, the weekly measurement of the residual sugar content in a liquid medium showed that root cells isolated from seedlings of the Jiromaru cultivar preferentially utilized fructose [104]. Therefore, these authors used 1% fructose (29.21 mM) in further studies (Table 1) [70]. However, in TCL explants of the Carpo and RZ1 cultivars, sucrose at 2% favored regeneration, while fructose impaired TCL development, although SE regeneration was observed [101].
Vitamins are also obligatory components of the medium. For the induction of somatic embryogenesis in spinach, some studies used an MS formulation of vitamins [96,101], while other studies enriched media with a range of B vitamins: 0.4–2 mg/l thiamine (B1), 0.5–2 mg/L pyridoxine (B6), and 0.5–5 mg/L nicotinic acid (B3) [69,97,98,99]. Xiao and Branchard [96] supplied 0.01 mg/L biotin (B7). Other adjuvants such as glutamine [96] or coconut water [53] were rarely used.
In addition to the standard media constituents, several other compounds have been shown to have a significant effect on the embryogenic response of spinach root apices. These compounds include hygromycin B (Hyg), trichostatin A (TSA), and dimethyl sulfoxide (DMSO). Hyg, an antibiotic commonly used to select genetically transformed cells, significantly promoted somatic embryogenesis from root apices and secondary somatic embryogenesis when administered at low doses of 0.5–1.0 mg/L [108]. As mentioned earlier, spinach roots are the preferred explant type for efficient SE induction. However, spinach root apices are fragile and tend to become necrotic during the selection of transformed cells (Milojević et al., unpublished results). Therefore, stepwise selection offers the possibility of improving the probability of the recovery of transformed SEs [108]. However, similar enhancement of the embryogenic response by Hyg was not observed in the leaf explants of in vitro spinach plants, presumably because only a high concentration of 20 mg/L was used for the selection of transformed cells [123].
In a preliminary study, promoting effects of TSA, an inhibitor of histone deacetylases, and DMSO, a widely used solvent, on SE initiation were observed [124]. DMSO at a concentration of 0.05% significantly enhanced SE induction compared with the control, probably by enhancing the uptake of NAA and GA3 into plant cells, whereas the enhancement of the embryogenic response obtained with 0.1–0.5 μM TSA suggests a significant role of histone acetylation in the epigenetic regulation of SE induction.
2.6. Culture Conditions
The effects of culture conditions have been studied only to a limited extent in spinach. In most studies, cultures have been exposed to diffuse light provided by cool white fluorescent tubes under a long-day photoperiod (LD, 10–16 h of light) and a photosynthetic photon flux density (PPFD) of 20–250 μmol m−2 s−1 from the beginning of culture [69,84,97,98,99,100,104]. Only Xiao and Branchard [96] and Leguillon et al. [101] kept cultures in the dark for the first 1 and 2 weeks, respectively, after culture initiation.
However, when root apices of the same seedling were divided into two groups, one of which was exposed to LD conditions (16 h photoperiod) and the other to short-day (SD) (8 h photoperiod) conditions, the LD condition was always favorable for the induction of SEs [109]. Explants from 7 of the 40 lines tested were able to regenerate SEs only under LD conditions. Moreover, regeneration under SD conditions was delayed by four weeks in all lines compared to LD conditions. Moreover, the embryogenic response of root apices of SE-derived plants was most efficient at a PPFD of 100 μmol m−2 s−1 [109]. In an opposite study, SD was found to be more effective in promoting shoot bud regeneration from spinach cotyledons [82]. The discrepancies between the two studies may be due to the use of different explant types and/or genotypes, as Geekiyanage et al. [82] used a different cultivar and did not examine the response of individual seedlings under either light regime.
Ambient temperature has a strong influence on the de novo shoot regeneration of spinach leaves [123]. Regeneration efficiency was 3–4 times higher in leaf explants cultivated at 14 °C than at 20 °C and 25 °C among different cultivars.
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae9091048
This entry is offline, you can click here to edit this entry!
