Membranes have a broad range of applications in industry and medicine. One common application is the use of membrane processes such as diffusion and ultrafiltration in a blood purification technique called CRRT (continuous renal replacement therapy) in patients with AKI (acute kidney injury). A porous hollow-fiber polymeric membrane is used to remove from patients’ blood inorganic solutes, organic uremic toxins (Table 1), excess water, and in some cases like septic shock, the overproduction of cytokines. A distinguished feature of this therapy is a prolonged blood-membrane contact for up to 72 h. After that, the whole set used for CRRT needs to be changed.
The harmful effects of biofilm in healthcare-related situations include not only the insufficient regulation of serum levels of solutes due to permeation flux decline and selectivity failure, but also other adverse effects in the patients. In order to prevent biofilm formation, the membrane’s properties should be better tailored e.g., by modifications during preparation.
2. Protein Adsorption onto the Membrane Surface
The clinical performance of biomedical devices is limited by the contact of protein and cells with the surface. In particular, HD (hemodialysis) membranes are subject to dynamic interactions between plasma proteins and the membrane surface: a process involving constant adsorption and desorption as a result of hydrophobic interactions and hydrogen bonds, electrostatic, ionic and Van der Waals forces
[8].
In a process called the Vroman effect plasma proteins reversibly adhere to the surface and commonly get replaced in time by different types of proteins. Attached particles create a fouling layer with a complex and constantly evolving composition, so it is impossible to determine the structure of a given surface in detail
[9][10]. The thickness varies between 2 and 10 nm, while the concentration of proteins on the surface can be 1000-fold greater than the concentration of proteins in plasma. It limits the effectiveness of diffusion and convection processes and reduces solute removal, especially the clearance of medium and large molecules
[8][11].
The first proteins to adsorb onto the membrane surface are the ones most abundant in blood. Among them, albumin and fibrinogen are considered to be molecules that initiate layer formation. Their content in the fouling layer gradually decreases as they get replaced by coagulation factors from the contact pathway including factor XII, high molecular weight kininogen, prekallikrein and factor XI. This binding onto the membrane is competitive and makes proteins undergo conformational changes which uncovers access locations for blood cells or proteins thus further promoting adsorption
[8][11].
In protein adsorption, the surface chemistry and physical properties of biomaterials play a significant role. It appears that the Vroman effect is independent of flow and is most evident on negatively charged hydrophilic surfaces, however bonds with proteins are weaker in comparison with hydrophobic surface. A hydrophilic surface is less prone to protein adsorption showing anti-fouling properties
[8][11].
3. Bacteria Adhesion
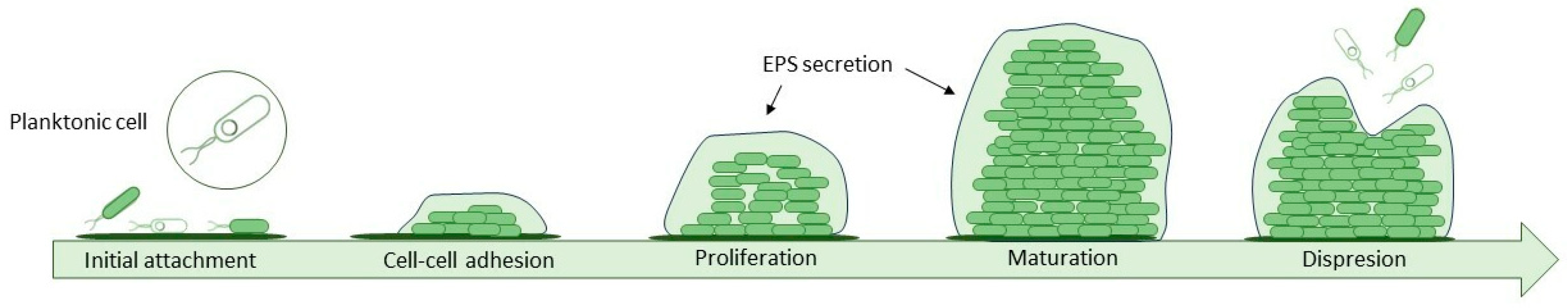
Proteins and chemical compounds suspended in the feed attach to the membrane's surface changing its properties to favour the adhesion of bacteria. This formation of a conditioning film is the first phase of biofilm formation, a complex process that can be described in five steps (
Figure 2). On such adjusted surface, cells are easily deposited. Once they settle, they start to organize into microcolonies and secrete an extracellular polymeric substance (EPS) which provides structural integrity by irreversibly bonding cells and offering them protection. It consists of a mix of polysaccharides, proteins, D-amino acids, fatty acids and a variety of nucleic acids and accounts for 80–90% of biofilm mass. Over time biofilm grows thicker and newly deposited layers vary in bacteria species, composition and oxidation level. The last step, when bacteria cells located in the top layer get dispersed back into the surrounding fluid, is called sloughing
[12][13][14].
Figure 2. Five steps of the biofilm development process.
Biofilms are multi-species. The microbiological component of the biofilm consists of Gram-positive bacteria, Gram-negative bacteria and fungi. For medical devices, representative pathogenic Gram-positive species include:
Staphylococcus aureus,
Staphylococcus epidermidis,
Enterococcus faecalis and
Streptococcus viridans and Gram-negative ones including
Pseudomonas aeruginosa,
Escherichia coli,
Klebsiella pneumoniae and
Proteus mirabilis [13][15]. Studies on strains of pathogenic bacteria like Staphylococcus (
S. aureus,
S. epidermidis) and Streptococcus showed structures expressed on bacteria membranes called microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) which are responsible for the ligand-receptor binding with plasma proteins, platelets or other cell of a given tissues
[16].
The feed is the main source of bacteria and the rate of biofouling in the cross-flow filtration does not change whether the dragged bacteria are dead or viable
[6]. However, the contamination may come from medical professionals during placement procedures or from the patient’s skin
[15]. The type of bacteria also depends on the drugs administered through the catheter, e.g., during the infusion of catecholamines Gram-negative bacteria are more often isolated
[17].
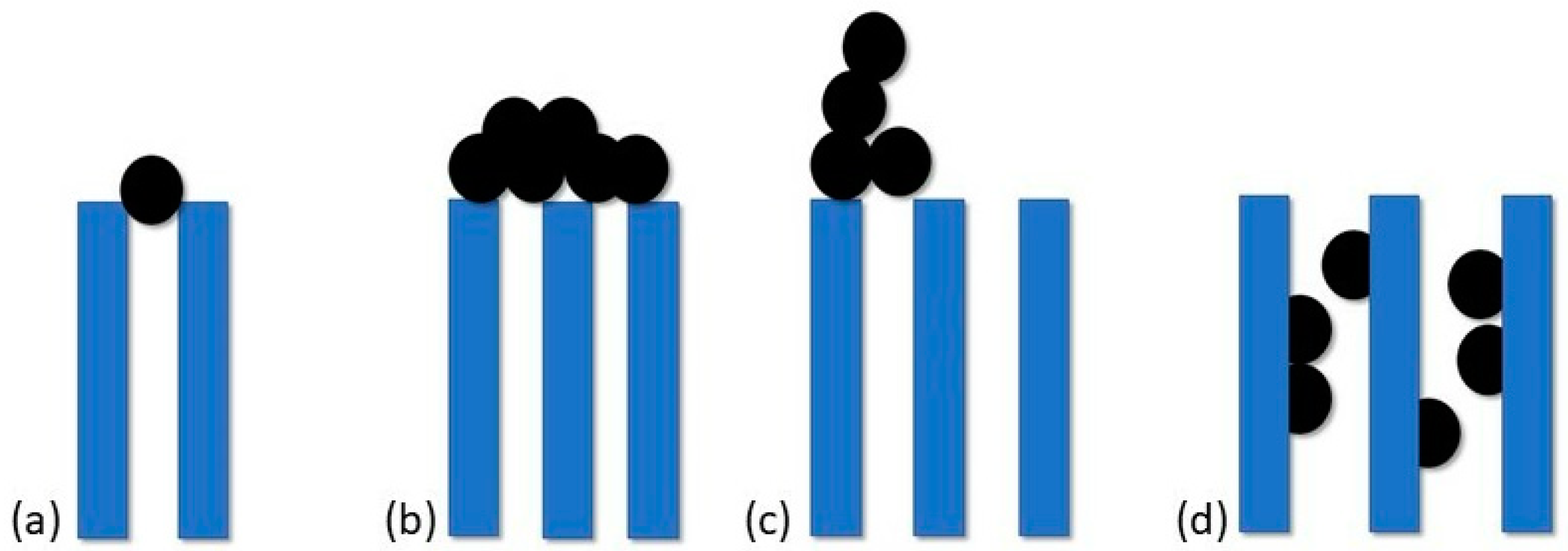
Several factors influence the rate of cell attachment, including the number and type of cells suspended in the fluid, the flow rate through the device, and the physicochemical properties of the surface
[15]. Studies have shown that the formation of a biofilm begins rapidly, after about 1 min of immersion of the surface and because bacteria have a negative surface charge, especially in the early phases of the cell cycle, this process occurs faster on positively charged surfaces. Therefore, the first phase of adhesion depends mainly on hydrodynamic and physiochemical processes like electrostatic forces, van der Waals forces and acid-base interaction based on Lewis theory
[14][18].
The second phase is irreversible and can take several hours depending on the species. An important role played by biological processes and changes in bacteria metabolism is associated mainly with different gene expression and the production of novel proteins. For example, in
Pseudomonas fluorescens a new ABC transporter and a secretion of proteins are required for irreversible attachment to occur
[14][19]. In addition, the same species show different properties, physiology and gene expression depending on whether they are planktonic or biofilm-forming
[20]. In the human environment, bacteria can incorporate host components such as immunoglobulins, platelets or fibrin into the biofilm matrix
[21]. The composition of the biofilm changes dynamically over time: the pioneer bacteria first to adhere may be displaced by subsequent species or disappear over time, leaving behind a rich surface ready to accept succeeding colonizers.
4. Interactions with Blood Cells
It is hard to describe blood cell-membrane dynamic interaction in chronological order because many of these steps occur simultaneously and promote each other in a feedback loop. Among other cells, platelets tendency to adhere to biomaterials causes their activation and degranulation, what can further enhance cell attachment.
RGD peptide is an amino acid sequence (arginine-glycine-aspartate) mediating the attachment of numerous cell types to the surface of biomaterials. Integrins are transmembrane receptors responsible for cell adhesion, which recognize RGD sequence in ECM proteins such as fibronectin, vitronectin, fibrinogen or osteopontin and bind with them initiating the aggregation process
[22]. An example of integrin expressed on a platelet membrane is very late antigen 5 (VLA-5) and GP IIb/IIIa, which binds RGD sequences of fibrinogen and fibronectin. Under static and dynamic conditions RGD is a crucial initiator of platelet deposition and the research confirms a direct correlation of fibrinogen adsorption with consequent platelet adherence onto artificial surfaces
[23][24].
Activated platelets change their shape and release α-granules filled with fibrinogen, β-thromboglobulin, thrombospondin, vWF and fibronectin, substances that are procoagulant in nature and further stimulate thrombus formation
[23]. Various pathways are likely to contribute to platelet activation, some of which are more relevant in the time of high complement and leukocyte activation and others under different health conditions.
Another cause of clotting is a bacteria-platelet interaction where binding takes place thanks to released ESP. Also, during an infection, a thrombus may form as a secondary effect of accompanying systemic platelet activation and DIC (disseminated intravascular coagulation). Some processes may even lead to the internalization of bacteria by platelets. The first description of this phenomenon comes from studies on
S. aureus stimulated by ADP
[16].
Surface-induced thrombosis occurs without a coagulation cascade in the absence of thrombin. On most polymer or metal surfaces where fibrinogen is readily adsorbed fibrin formation is spontaneous. This process requires a specific orientation of fibrinogen molecules: on the hydrophilic surfaces it takes a globular form, whereas on the hydrophobic surfaces, large fibers
[25]. As proteins bind to a surface, platelet adhesion is promoted, which mediates further blood clotting.
These processes are responsible for the thrombogenic properties of medical devices and further modification of the surface is needed to eliminate each one as the potential cause of biomaterial-related thrombosis.
5. Methods of Anticoagulation
Blood-contacting devices are prone to protein fouling that initiates a coagulation cascade and results in thrombus formation. In the case of HD, constant filtration of blood makes membranes even more susceptible to pore-blocking and may cause abrupt and complete clotting of the hemofilter. Excessive thrombus formation is controlled by the use of anticoagulation. It allows for longer patency of vascular access, but also a longer lifespan of the membrane.
The key to proper anticoagulation is to maintain a balance between thrombus formation and excessive bleeding in patients which may lead to further adverse events like hemorrhagic stroke and internal bleeding. Strategies include the use of heparin, both unfractionated and low molecular weight, regional citrate anticoagulation and novel membranes coated with heparin (e.g., oXiris)
[26]. In some patients with initial coagulation disorder, there is a possibility to carry out CRRT without any anticoagulants. Every method has its indication and contraindication and should be adjusted to the patients’ general condition and doctors’ experience.
Unfractionated heparin (UFH) is made up of heparin molecules of different sizes between 5 and 30 kDa. It works by inhibiting factors IIa (thrombin) and Xa of the coagulation cascade which stops thrombus formation. During treatment, it is critical to monitor activated partial thromboplastin time (APTT) closely and obtain recommended values between 35 and 45 s. The APTT is a good predictor of filter clotting and hemorrhage in patients: studies have shown that UFH prolongs filter life proportionally to the APTT but not to the given dose of anticoagulant
[27]. Heparin plasma half-life can extend up to 3 h with kidney injury which causes shifting in dosage and may cause unpredictable heparin blood levels further increasing the risk of hemorrhage. The incidence of bleeding events ranges from 10 to 50%, with mortality as high as 15%
[28].
Citrate anticoagulation is a safe and effective alternative to heparin, it prolongs hemofilter patency and reduces bleeding complications (notably less bleeding and less blood transfusion in comparison with heparin) in critically ill patients
[29]. Calcium ions are a coagulation factor IV that works in the last stage of the intrinsic and extrinsic coagulation cascades. Citrate binds and chelates free ionized calcium forming citrate–calcium complex, interrupting coagulation and thrombus formation.
Calcium is lost as the citrate–calcium complex via dialysis and filtration because it has a molecular weight of approximately 300 Da and can pass easily through the membrane. For that reason, the blood level of calcium ions must be restored before purified blood re-enters the circulatory system to ensure physiological systemic coagulation. Circuit’s and patient’s ionized calcium levels are measured frequently to adjust the dosage and guarantee efficient anticoagulation. The side effects are mainly connected to citrate accumulation and include metabolic alkalosis, metabolic acidosis, hypo- or hypercalcemia, hypernatremia and hypomagnesemia
[26].
6. Methods for Analyzing Biofilm on Membranes
The levels of planktonic cells do not correlate with the scale of biofilm formation
[19]. This is a reason why standard tests such as bacterial culture do not reflect the true extent of the biofilm and its biodiversity. Accumulation of ESP makes this analysis even more difficult by binding bacteria with each other. In addition, during the maturation stage, the biofilm grows thicker, which means that lower-lying species may also not be identified. There are a few methods that can confirm only the presence of biofilm and some, like flow cytometry, can give an exact number of detected bacteria, dead and viable, in one millilitre of the studied solution. To gather a full range of data we need to use a variety of methods, but we can select a technique dedicated to measuring exactly the requested biofilm parameters. The most accurate approaches include flow cytometry (FCM), X-ray photoelectron spectroscopy (XPS), time-of-flight SIMS (ToF-SIMS), SEM (scanning electron microscopy) and 16S rRNA sequencing.
7. Characteristics of Membranes for CRRT
A polymeric hollow-fiber membrane is the most prevalent choice in the contemporary hemofilter production. It is characterized by high separation area, high permeability, high selectivity and excellent mass–transfer properties
[30]. Hollow fibers have advantages over flat sheet membranes that ensure consistency of the purification process. They show better mechanical strength, larger pore areas with uniform size dispersion and low cost of production
[31][32]. Membrane properties are also affected by the topology, the shape of the pores and the general porosity
[33].
The surface topography may present antagonistic features that promote or mitigate adhesion and biofilm formation. As a consequence, the materials selected for hemodialysis membranes require deep consideration and precise testing of their biocompatibility. The first hemodialysis membranes were made with cellulose acetate but due to small pore size, complement activation and other side effects in patients, they are no longer commonly used. Novel polymers can withstand higher transmembrane pressure and do not ignite the inflammation response in patients. Among them are polysulfone (PSf), polyethersulfone (PES), polymethylmethacrylate (PMMA), ethylene vinyl alcohol (EVOH) and polyacrylonitrile (PAN). The overwhelming majority, as much as 93%, are derived from the polyarylsulfone family
[31]. PSf membranes are preferred because of their chemical inertness and mechanical strength. They also show high thermal stability and can endure all sterilization techniques
[34].
Using the phase inversion technique we are able to obtain asymmetric membranes with complicated porous structures divided into a skin layer and a support layer
[30]. A skin layer, also called an active layer, is a blood-contacting side. Its thickness is a determinant of diffusion efficiency, with an inverse relationship between these two parameters
[33]. The size of pores in this layer is also an eliminating factor for the size of removed molecules. The role of a support layer is to provide mechanical strength for the layer above, but also for the whole hollow-fiber structure that needs to withstand high-pressure differences during CRRT therapy.
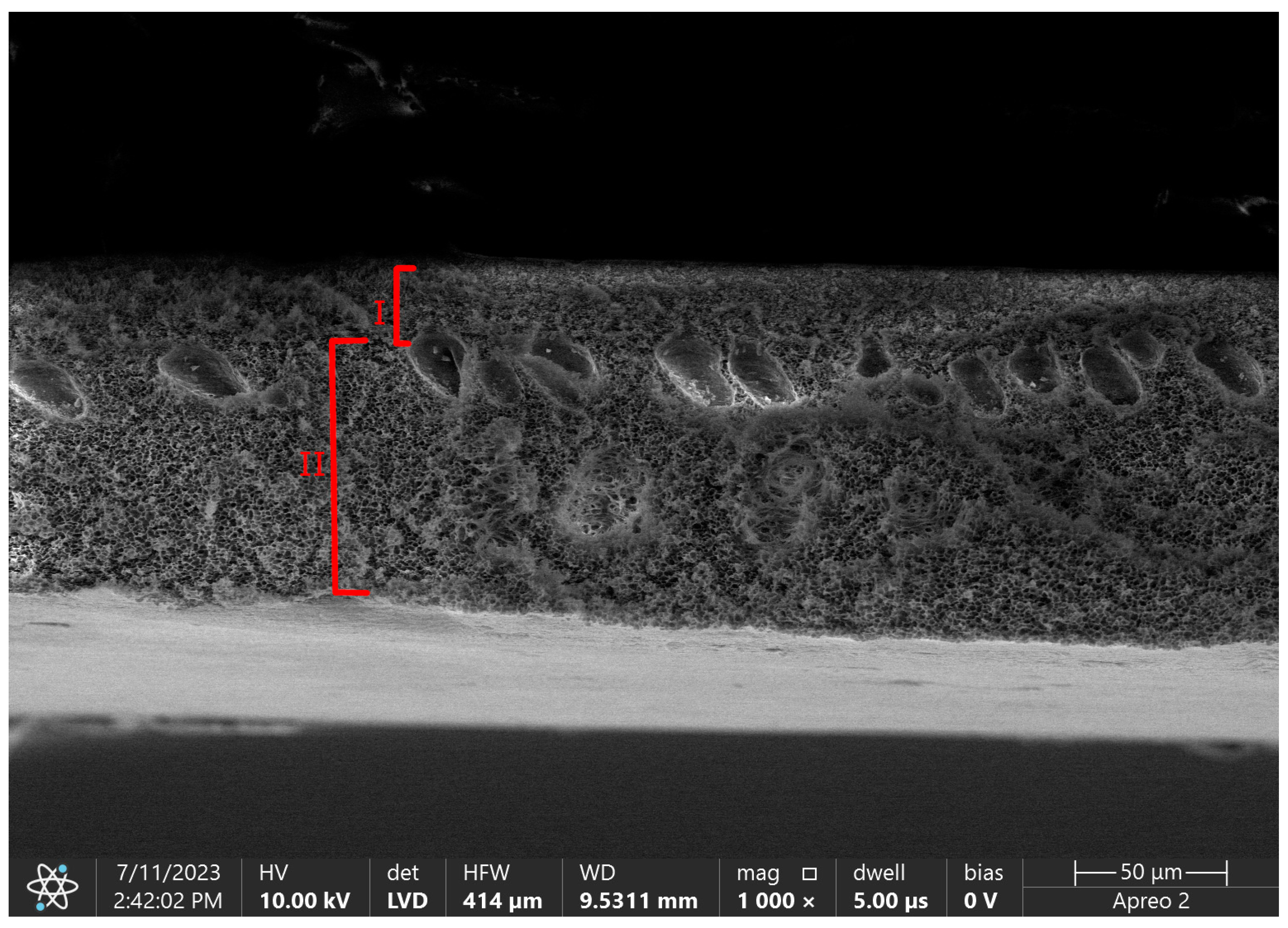
Figure 3 shows the difference in porosity between those layers in pure polysulfone flat sheet membrane.
Figure 3. SEM micrograph of flat sheet pure polysulfone membrane with marked layers. I—skin layer, II—support layer. Photo from own research.
One of the most crucial surface properties affecting biofilm formation is hydrophilicity. Hydrophilic surfaces show reduced protein adsorption and less nonspecific bacteria adhesion
[31][35]. Additionally, due to reducing hemofilter performance and a decline in flux, protein adsorption on the membrane also affects its hemocompatibility. Upon the adsorption of coagulation factors onto the artificial surface, the proteins undergo conformational changes or denaturation, which promotes thrombus formation
[1].
Another important factor is surface roughness. Greater roughness means a bigger surface area, which results in more sites of adhesion
[18]. The roughness of the surface enhances its inherent wettability properties: increasing the surface area of hydrophobic materials makes it even more hydrophobic
[36]. In one study, the contact angle was increased from 99.3° to 151.6° by increasing the roughness of polytetrafluoroethylene (PTFE) membranes, which showed a dual-reversible transition of wettability: upon alcohol prewetting or drying, the film could reversibly switch between superhydrophobicity and superhydrophilicity
[37].
The adherence of bacteria and blood cells to polymeric biomaterials depends on surface chemistry and morphology. Other factors, such as plasma proteins, platelets, and fluid pH should also affect bacteria adherence
[8]. Although over the years hemocompatibility aspects were improved, not so many advances were made in the field of fouling prevention. Due to its irreversible nature, the main emphasis should be put on methods mitigating biofilm formation and numerous modifications have already been attempted. The variety of methods includes polymeric blending, surface chemical modification, coating, grafting surfaces with heparin and hydrogel surface modification. It can also be an approach based on nanoadditives like carbon nanotubes (CNTs), graphene and nanosilica. Adding these nanomaterials to polymer solution prior to casting the film employs their antimicrobial properties to limit biofilm formation and enhances the existing properties of membranes.