Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Environmental Sciences
Piezocatalysis is a technique that relies on the conversion of mechanical energy to trigger chemical reactions that can be utilized to catalyze the degradation of pollutants in water. By combining piezoelectric materials with photocatalytic materials, the built-in electric field generated by the piezoelectric effect promotes the separation and migration of photogenerated carriers, thereby enhancing the efficiency, selectivity, and stability of photocatalytic reactions.
- photocatalysis
- piezo
- wastewater
- environmental engineering
- materials
1. Photocatalysis
1.1. Overview
Photocatalysis is a process of solar energy conversion [26]. It involves the use of light energy to activate a photocatalytic material that has mostly been explored as semiconductors such as TiO2 [5], BiVO4 [27], spinel compounds [28], metal-free materials such as g-C3N4 [29], and recently developed natural materials such as pollen [30,31]. Electron–hole pairs are generated in both the material bulk and surface under solar irradiation. These charge carriers can then participate in redox reactions with pollutants and degrade them into harmless substances. Photocatalysis is an ecofriendly technique that has many advantages over conventional methods, such as low cost, high efficiency, and protection of treated water from secondary pollution.
However, there are also some challenges that limit the industrial application of photocatalysis, such as low visible light utilization, fast recombination of electrons and holes, and low migration ability of the photogenerated electrons and holes from the bulk to the material surface [26]. To overcome these challenges, various strategies have been developed to modify the photocatalysts and enhance their performance. Some of these strategies include doping with metals or non-metals [32], coupling with other semiconductors [27,33], loading with co-catalysts [34,35], embedding quantum dots [36,37], and incorporating conductive films and flakes [38,39]. Construction of two-dimensional layered or hollow configurations enhances the utilization of sunlight in material composites (graphene, g-C3N4, two-dimensional flake MoS2, etc.) [10,40,41]. These methods mainly improve the charge separation efficiency and, at the same time, provide more reactive sites for light absorption and charge migration. The photocatalyst activity is subsequently enhanced, extending the application horizon of this technology. Photocatalysis has been applied to treat various types of emerging pollutants in wastewater, such as dyes, pesticides, pharmaceuticals, oil, grease, and textile effluents [42]. Photocatalysis can also be integrated with other processes, such as membrane filtration [43], to achieve higher removal efficiency and water quality. Although photocatalysis is a promising technology that can contribute to the sustainable development of environmental remediation and energy production, the limits of this technique in practical applications are yet to be conquered.
1.2. Improvement Strategies
Research has been conducted to summarize and evaluate diverse materials for photocatalysis. TiO2 is the most widely explored material due to its outstanding characteristics [14], and trial of its application in the real world has been reported [19]. However, limited reaction to a wide range of the solar spectrum constrains the practical potential of TiO2. Therefore, modifications have been developed to push the broader future of TiO2. Element doping is a convenient method used to introduce foreign materials and structures into semiconductors. Doping metal and non-metal elements in TiO2 refers to the introduction of foreign atoms into the crystal structure during the synthetic process, which provides an intermediate state in the band gap and transit center for the electron–hole pair, thereby affecting the generation of reactive species by tuning the redox potential and narrowing the band structure, which allows a higher response to the visible light of solar energy [32,44]. However, the doping sites are also the recombination centers for electrons and holes; thus, balance between insertion of the mid-gap in the lattice and the introduction of recombination centers is a concern when applying the doping method [45]. Crystal facet design is also a convenient method, but transit of different facets of semiconductors always occurs over time, so maintaining a stable and effective facet is a challenge for long-term use [46]. Moreover, engineering the heterojunction structure is a widely accepted strategy. The diversity of materials provides not only a variety of material characteristics but also facile synthesis process design, which further benefits band structure engineering involving solar energy harvesting [47]. A similar mechanism is applied to engineer photocatalysts through the deposition of noble metal such as Ag, Pt, and Au [34,48].
Furthermore, due to the unique optical and electronic properties owing to their size-dependent quantum confinement effects, quantum dot photocatalysts exhibit the ability to absorb incident light and generate electron–hole pairs, which subsequently participate in redox reactions with target molecules or species. The performance of quantum dots as photocatalysts can be finely tuned and optimized, holding considerable promise for environmental remediation strategies. Malile investigated the photocatalytic degradation of organic pollutants using Mn2+-doped CdS/ZnS with a core–shell quantum dot structure [49]. Reduction of nitrobenzene was completed in 15 min under 395 nm irradiation, and the compound could retain consistent efficiency over a 5-cycle reaction trial. Known for their excellent optoelectronic properties, the combination of quantum dots and perovskites, such as cadmium telluride quantum dots on perovskite cells [50], is encouraging due to their excellent solar conversion. In addition, perovskites suffer from toxicity concerns due to the presence of lead; thus, lead-free perovskites are attracting significant attention as alternatives to traditional lead-based perovskites as an eco-friendly approach.
Briefly, introducing foreign materials to semiconductors can modify the band structure, which improves energy harvesting, and can enhance the migration of charge, which involves higher reaction efficiency.
2. Piezocatalysis
2.1. Overview
Piezo-based catalysis is a process applying the piezoelectric characteristic of converting mechanical energy to electricity, which subsequently initiates the redox reaction by acceptance and donation of elections. Piezoelectric techniques for environmental pollution remediation can be divided into three types: piezocatalysis, piezo-electrocatalysis, and piezo-photocatalysis. Piezocatalysis refers to the direct degradation of pollutants by the piezoelectric potential generated under mechanical stress. Piezo-electrocatalysis refers to the enhancement of the electrocatalytic reaction by the piezoelectric potential under mechanical stress and external bias. Piezo-photocatalysis refers to the synergistic effect of piezoelectric potential and photocatalytic reaction under mechanical stress and light irradiation. These techniques have been applied to the removal of various pollutants, such as dyes, phenols, pesticides, herbicides, heavy metals, and radionuclides.
Piezoelectric materials are characterized by their ability to generate electrical energy when subjected to mechanical stress or deformation. These materials have been widely investigated and utilized in the development of various devices, including sensors, actuators, and energy-harvesting systems. In recent years, piezoelectric materials have also been explored for their potential use in catalytic applications, particularly in tackling environmental problems. Due to the built-in electric field, piezo materials excite electrons under mechanical vibration for the subsequent redox reaction of electrons and groups in water. In this way, radicals and non-radicals are generated to initiate wastewater treatment reactions such as emerging pollutant removal. Piezoelectric materials have recently been developed from simple single-crystal quartz to perovskites, such as BaTiO3, PbTiO3, zinc oxide (ZnO), two-dimensional layered molybdenum disulfide, and organic polymers such as polyvinylidene fluoride (PVDF) [51,52]. In 2006, Wang et al. first demonstrated that the piezoelectric material ZnO, in the form of nanowires, could convert mechanical energy into electrical energy under external mechanical force, generating a self-polarizing electric field within the material [53]. An electrical signal was detected when the nanowires were deformed, unveiling their characteristic as an electricity generator. The successful conversion of nanoscale mechanical energy into electrical energy results in the initiation of the redox reaction aimed at pollutant degradation. When the bias potential introduced by the piezoelectric effect is beyond 3 volts versus the standard hydrogen electrode, the materials are capable of initiating the redox reaction [54]. The work performed by Hong et al. started a new chapter for piezocatalysis in the environmental treatment field. They applied the piezoelectric effect of BaTiO3 micro-dendrites under ultrasonic vibration to degrade Acid Orange (AO7) dye in aqueous solution [55]. After bending caused by vibration, BaTiO3 dendrites yielded bias potential, and oxidation and reduction reactions occurred by accepting electrons and positive holes, respectively (Figure 2). Subsequently, reactive oxygen species were generated to degrade AO7, obtaining a degradation kinetic constant of 0.50 mg/L/min with the adsorption constant of 0.149 (mg/L)−1, which revealed an efficient catalytic process. This work broadened the horizon for environmental approaches.
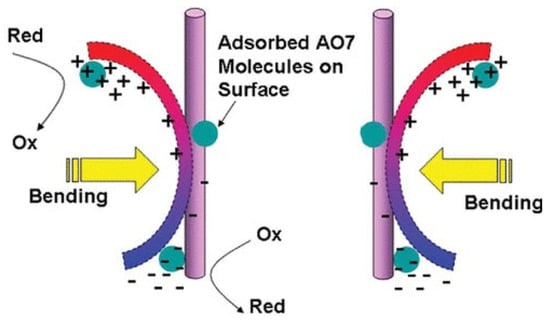
Figure 2. Schematic demonstration of redox reaction initiated by the piezoelectric effect for organic pollutant degradation [55].
In lab-scale studies of piezocatalysis for pollutant removal in water and wastewater, the mechanical source is normally provided by ultrasound, which simulates natural vibration. The influence of the energy source on piezo material performance is still unclear in the few studies reported. The point is worthy of exploration in paving the way for practical applications of the piezo technique. Zhang et al. [56] and Bößl et al. [57] studied the effect of frequency and power on the degradation of organic pollutants in water. Similar conclusions were obtained that degradation efficiency improved with increasing frequency and power, at a frequency lower than 600 kHz. As frequency further increased to around 1000 kHz, the degradation efficiency gradually declined. When the frequency was 20 kHz, the degradation rate increased with increasing power. Moreover, Bößl et al. found that at a high frequency of more than 100 kHz, the degradation was derived from acoustic cavitation [57]. Therefore, piezocatalysis is unnecessary at high ultrasonic frequencies. However, this does not raise questions about the benefits of utilizing the piezo technique. The principle of catalysis is to apply natural energy for organic removal, whereas the extreme condition of high frequencies is rarely seen in the environment.
2.2. Properties and Applications in the Environmental Field
Researchers have been developing piezo materials for environmental protection applications. Piezoelectric materials have recently been developed from simple single-crystal quartz to perovskites, such as BaTiO3, PbTiO3, zinc oxide (ZnO), two-dimensional layered molybdenum disulfide, and organic polymers such as polyvinylidene fluoride (PVDF) [51,52]. Perovskite is a promising material in the energy field and attracts intensive attention globally. As a milestone of piezo materials, BaTiO3 possesses the ferroelectric characteristic of perovskite materials with a structure of ABO3, as perovskite materials have high piezoelectric performance [55]. Having a similar formula and structure, PbTiO3 is another attractive piezo material that shows several times higher effects [58]. A simple hydrothermal method was used to synthesize piezo particles with surfactants to enhance the degradation of dyes. Amiri et al. utilized ultrasound to cause PbTiO3 deformation [59]. Under 18 kHz ultrasonic vibration that simulated natural vibration, reactive oxygen species of •O2−, •OH, and H+ were generated to remove 69.7% of acid red dye and 96.5% of acid black-2 dye (5 ppm) after 30 min, respectively. The differential preference was likely due to the naphthalene position in the dye formula. Therefore, in the investigation of the piezo effect of materials for organic pollutant degradation, efficiency is not the only concern when evaluating the materials, but also the reactive oxygen species (ROS) generation and their specific role in targeting functional groups and chemical compounds of the pollutants. In this way, a more mechanism-oriented logic rather than result-oriented material design can be obtained. Other than developing material design, strategies to improve the catalytic capacity by modifying the material’s compound structure are also widely explored. Regarding structured perovskite materials, Xu et al. formed layered Bi2WO6 for catalysis reactions, indicating that the special structure enabled a comparable activity to the typical perovskite structure [60]. Other widely explored perovskite piezo materials are BiFeO3 [61], PbZrO3 [62], PbTiO3 [59], NaNbO3 [63], SrTiO3 [64], etc. These are artificial oxide materials with tunable structures for facile application. Similar perovskite structures provide fast response to vibration under a natural environment for the redox reaction and organic pollutant removal. Furthermore, You et al. discovered an organic-inorganic crystal material, trimethylchloromethyl ammonium trichloromanganese (II), which exhibited a competitive piezoelectric coefficient to that of BaTiO3 [65]. It had the advantages of being lightweight and mechanical flexible and could be processed at low temperatures via an environmentally friendly method, arousing the interest of researchers. Although perovskite materials are a promising option for the piezo reaction, drawbacks such as instability in the liquid/humid environment limit their application in environmental situations [66]. The discovery of various materials with piezoelectric characteristics provides opportunities to harvest natural energy for environmental applications.
In particular, metal oxides are shining stars on the stage of piezo materials. Wang et al. introduced ZnO nanowires as electric microgenerators under mechanical deformation, pointing to their future in the application of natural energy sources for chemical reactions [53]. Xu et al. further released electricity generated by vibration to initiate chemical reactions, thus decoloring wastewater in 50 min with the formation of hydroxide radicals [67]. Vibration-excited ZnO nanorods generated charge carriers, which subsequently reacted with OH− and O2 to form reactive oxygen species and then initiated the degradation of AO7 (Figure 3a), exhibiting a removal efficiency of 80% AO7 after 50 min. The reaction efficiency of the piezo ZnO nanorods was promising for the purification of wastewater (Figure 3b). Bismuth oxide-based materials are other types of piezo materials with simple structures. Chen et al. combined Bi5O7I with Ag nanoparticles to form a composite structure using a facile hydrothermal calcination technique [68]. Bi5O7I nanorods were prepared using the hydrothermal method with subsequent photo-deposition of Ag nanoparticles. As the Ag nanoparticles trapped electrons generated in the piezoelectric process of Bi5O7I, charge carrier separation became more efficient for reaction. However, vibration may have dragged the Ag nanoparticles away from Bi5O7I and destroyed the composite structure, resulting in decreased performance after the first cycle [68].
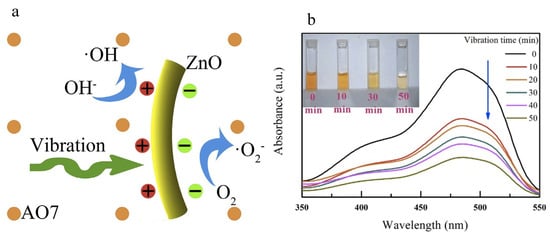
Figure 3. (a) Basic principle of the vibration-catalytic process; (b) Decoloring AO7 in wastewater using ZnO nanorods for different vibration times [67].
The practical potential of utilizing the piezo reaction requires the feasible fabrication of piezo devices. For the effective harvest of mechanical energy, a layer with a sufficient surface area is suggested for piezo materials. The polymer seed layer is a nanoconstruction method for piezo films. A polymeric seed underlayer refers to a thin film or coating of a polymer material that is applied as a base or foundation layer in various thin film deposition processes. It serves as a template or “seed” upon which subsequent layers are deposited. Aleksandrova [69] used this approach to synthesize a Ga-doped ZnO piezoelectric film. The dendrite structure provided the film with a larger specific surface area and higher efficiency of energy harvesting. Moreover, according to the application scenarios, structured piezo materials are also obtained using template-assisted approaches, e.g., using an Au array to form spatially separated ZnO pillars [70] and introducing template-assisted growth of piezo material KNbO3 in porous aluminum oxide [71].
Piezoelectric behavior is a property shared by several polymer families, including fluoropolymers, polyurea [72], polyamides [73], polypeptides [74], polysaccharides, and polyesters [75]. Many biopolymers, such as collagen [76], cellulose [77], and silk [78], exhibit piezoelectric characteristics. There is significant research on using piezoelectric materials, both natural and synthetic, in biological contexts [79]. In addition, polymer piezo materials attract attention due to their mechanical flexibility. Well-developed polymers, such as polyvinylidene fluoride (PVDF), polyparaxylene, poly-bis(chloromethyl)oxetane, aromatic polyamides, polysulfone, and polyvinyl fluoride, generate a built-in electric field under strain and structural deformation [80]. Notably, their lead-free character is promising in the environmental field. In the 1970s, it was discovered that PVDF, a semicrystalline homopolymer composed of vinylidene fluoride (VDF), has piezoelectric and ferroelectric properties. PVDF has five crystalline phases: alpha, beta, gamma, delta, and epsilon. The beta-phase of PVDF, with adjacent carbons in the trans conformation (carbon atoms are 180° apart), exhibits a ferroelectric nature and has the highest piezoelectric performance. However, its electromechanical performance is still lower than that of the typical Pb(ZrTi)O3 format. Targeting the low electromechanical performance of PVDF, Wang and Liao synthesized PVDF-based copolymers with enhanced piezoelectric performance [51], developing the copolymer shown in Figure 4a. One of the most researched options for PVDF is its combination with trifluoroethylene (TrFE). The larger size of TrFE limits the formation of adjacent carbons in the gauche conformation (carbon atoms are 60° apart) and encourages their trans conformation in the beta-phase, regardless of how it is processed. Additionally, a P(VDF-TrFE) chain has a higher proportion of crystalline regions than PVDF, resulting in a slight increase in k33 and d33 values to 37% and −38 pm/V, respectively. According to the mechanical flexibility of PVDF, Ma et al. employed another strategy to utilize PVDF in piezocatalysis [52]. Using an electrospinning process, a piezocatalytic membrane was created by combining exfoliated, multi-flawed, MoS2 nanosheets with a polyvinylidene fluoride (PVDF) matrix. The MoS2/PVDF membrane demonstrated a high antibiotic oxytetracycline degradation efficiency of 93.08% after 24 min of ultrasonic irradiation in the dark, exhibiting a pseudo-first-order kinetic constant of 0.09124 min−1, representing a 21.1 times increase in efficiency compared to a pure PVDF membrane. The embedded MoS2 nanosheets and high beta-phase proportion (94.1%) of PVDF synergistically contributed to this enhanced performance, which subsequently improved the piezoelectric property of the composite and promoted the generation of •OH and •O2– radicals for oxytetracycline degradation. After five consecutive cycles, the material exhibited sustained piezocatalytic activity, obtaining a stable removal efficiency of 92–93% under the same test conditions. Wu et al. developed a piezoelectric channel on a membrane for filtration [81]. Activated by ultrasound during the filtration process, piezoelectricity was generated by the MoS2-embedded PVDF film to trigger the peroxymonosulfate reaction, thereby removing emerging pollutants in wastewater. The efficiency of removal of carbamazepine was improved from 18.3% to 93.8% after 3 h of piezo-boosted reaction. The advantage of the designed filtration membrane was the stability of reaction performance after even 9 cycles. The membrane form of MoS2/PVDF allowed a facile fabrication. What’s more, the developed piezoelectric membrane could be applied in a conventional membrane filtration facility without extra renovation.
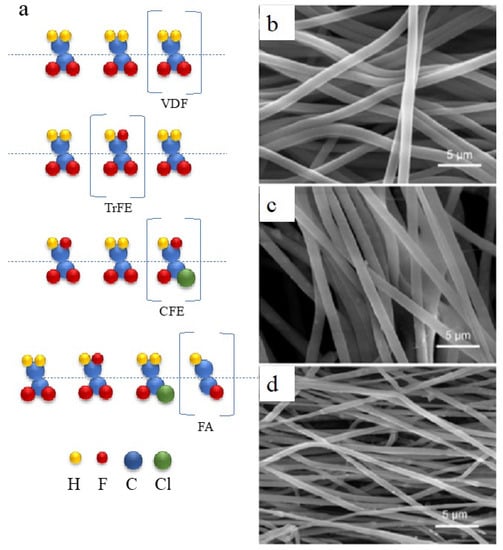
Figure 4. PVDF improvement strategies: (a) Different random copolymers of ferroelectric PVDF; (b–d) SEM images of PVDF fiber web with embedded thin-film MoS2 [52].
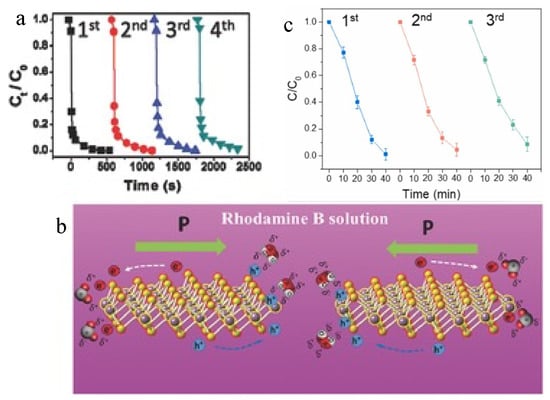
Other types of materials with attractive piezoelectric characteristics, such as MoS2, also show outstanding potential. Odd-layer MoS2 flakes synthesized by Fan et al. converted mechanical vibration energy from water flow to electricity as a nano electric generator, unveiling piezoelectric properties [82]. The performance of the layered materials surged when the layer number was decreased, in compliance with the outstanding catalytic performance of the thin-film MoS2 with less layer overlapping. The structure is a crucial factor in material characteristics, particularly in piezoelectric performance, as it is affected by the approach of harvesting energy. Nanoflower-like MoS2 was synthesized by Wu et al. [83] using a hydrothermal method to form single and few-layer structures (Figure 5a,b). They were subsequently applied for the degradation of RhB at a concentration of 10 ppm in water in an environment of ultrasonic vibration [83]. A reaction constant of 40,430 Lmol−1s−1 was obtained in the removal of RhB, which was the highest reported degradation efficiency targeting RhB in aquatic solution before 2016. Lee et al. [84] fabricated MoS2/carbon fiber utilizing the vibration of water flow in pipelines to purify wastewater. Due to the piezoelectric characteristic, charge carriers are generated in the bulk and surface of MoS2. Referring to the conductivity of carbon materials, charges on the MoS2 surface and interface of the composite easily migrate, subsequently inhibiting the charge recombination and leading to the improvement of catalytic efficiency. As the MoS2/carbon fiber was set in the pipelines, it acted as a built-in filter. When wastewater passed through the pipeline, it simultaneously decomposed the organic pollutants in wastewater through the piezo reaction driven by natural water flow. The MoS2/carbon fiber filter system completely degraded 1000 mL of RhB solution (10 ppm) after 40 min of circulatory reaction in the dark. A 3-cycle treatment of the RhB dye solution approached a decrease of total organic carbon by 90%. It maintained a stable efficiency under the same reaction conditions (Figure 5c), indicating the potential for scaling up the technique. It is interesting that the cycling performance of the piezoelectric reaction was stable, possibly owing to insignificant destruction of the piezo composite structure in the reaction. This finding revealed the high potential for practical applications of the piezo-relevant technique. Regarding the harvest of mechanical energy from water flow and waves, a flexible fiber-based piezo reactor was developed by An et al. [85]. It consisted of polymer nanofibers, poly (vinylidene fluoride-co-trifluoroethylene), and novel nanoparticles, barium strontium titanate (BaSrTiO3), embedded on the fibers. Incorporation of BaSrTiO3 nanoparticles enhanced the sustainability and enabled unique flexoelectricity-enhanced piezoelectric properties. The composite demonstrated excellent mechanical durability and cyclability. The design of the piezoelectric generator holds potential for converting water wave energy for environmental applications, contributing to the advancement of sustainable energy technologies and eco-friendly environmental treatment.
In the practical application of wastewater treatment, changes in ambient temperature can cause the materials to deform and stimulate piezoelectric reactions, thus converting mechanical energy into chemical energy and further expanding the sources of exploitable energy [86]. This validates the possibility of using piezoelectric materials to obtain mechanical energy and trigger electric fields in wastewater treatment. Piezoelectric reactions receive a continuous flow of mechanical energy from nature’s streams, offering the possibility of all-weather operation. However, due to poor response under light irradiation, they cannot take advantage of abundant solar energy.
3. Piezo-Photocatalysis
3.1. Overview
In the photocatalytic process, the transmission and recombination of charges and the high dependence on irradiation conditions have not been fundamentally resolved, and the practical feasibility is still limited. A more effective method is to apply a bias potential to the photocatalytic material to separate photogenerated carriers with an external electric field, so as to greatly improve the participation of electron–hole pairs generated by sunlight in photocatalytic conversion during chemical reactions and enhance catalytic reaction activity. The combination of electronic chemistry and photocatalysis is a way to achieve this target [87]. However, an external electric field requires additional power consumption and external electrochemical equipment, complicating the treatment process and increasing operating costs. Nevertheless, inspired by this principle of photoelectrocatalysis, the idea of integrating piezoelectricity with photocatalytic reactions has emerged.
Piezo-photocatalysis is a novel technique that combines the piezoelectric and photocatalyst properties of materials to enhance the reactive performance. In light of the aforementioned limitations of photocatalytic and piezoelectric catalytic reactions, coupling piezoelectric and photocatalytic reactions for the treatment of environmental issues can achieve three advantages. First, it can capture multiple forms of energy while converting the abundant solar and mechanical energy from nature. Second, the coupling can enhance reaction efficiency by using the piezoelectric built-in electric field to suppress photocatalytic photogenerated carrier recombination and promote photocatalytic efficiency. Third, the coupling can overcome the limitation of daylight hours and respond to both light and dark conditions. Piezo-photocatalysis has several advantages over conventional photocatalysis, such as low energy consumption, high selectivity, and wide applicability.
Piezoelectric materials are a promising avenue for improving the photocatalytic activity of semiconductors. A schematic illustration of the comparison of photocatalysis and piezo-photocatalysis under different natural energy sources is exhibited in Figure 6. The piezoelectric effect generates an electric field through mechanical deformation, which can shift charge energy levels or separate photogenerated charge carriers, ultimately enhancing the photocatalytic efficiency and facilitating the reduction of pollutants by creating reactive species [24]. The generation of an electric potential or field is dependent on the type and intensity of the mechanical energy applied to the piezoelectric materials. Various sources, such as vibration, ultrasound, and fluid flow, can induce different deformation modes, like bending, stretching, and twisting. This deformation leads to the polarization or displacement of ions or dipoles, creating a potential difference or an internal electric field across the material [88]. The interaction between electric fields and photocatalysts is a strategy employed to tackle the challenge of applying photocatalysis by affecting the electronic structure, charge dynamics, and surface chemistry. The electric field can shift the band edges, modify the band gap, or induce new energy levels in photocatalysts, altering their optical properties. It can also separate, transfer, or inject electrons and holes between different components or regions of photocatalysts, enhancing their charge utilization. Finally, the electric field can alter the surface potential, adsorption affinity, or reaction kinetics of photocatalysts, influencing their catalytic activity [89].
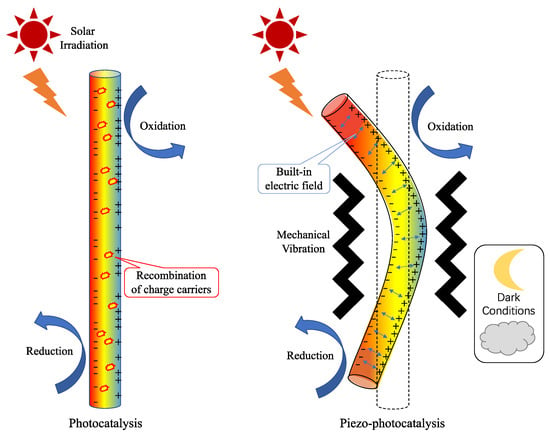
Figure 6. Schematic illustration of the mechanisms of photocatalysis and piezo-photocatalysis.
One of the significant advantages of piezo-photocatalysis is its high efficiency in degrading pollutants under different conditions. Since the technology can utilize both light and mechanical energy, it can enhance photocatalytic activity and facilitate the electron transfer process, leading to a faster reaction rate and higher degradation efficiency. Mechanical energy is induced in the natural environment to initiate the piezo reaction. Ultrasonic waves can create high-frequency pressure waves that result in the mechanical deformation of both the piezoelectric material and the surrounding water molecules, leading to enhanced pollutant degradation. Mechanical vibration, on the other hand, can create a similar effect by imparting mechanical stress on the piezoelectric material.
3.2. Synergistic Effect of Piezo-Photocatalysis
Regarding the materials, piezo-photocatalysts can be classified into two types: integrated piezoelectric semiconductors and coupled systems composed of piezoelectric materials and semiconductors. The former category includes materials such as BiOCl [90], KNbO3 [71,91], NaNbO3 [92], BaTiO3 [93], etc., which can generate internal electric fields under mechanical stimulation that modulate their band structures or surface states to facilitate charge separation or transfer. Several piezoelectric materials have been investigated for their photocatalytic applications, including piezoelectric ceramics such as Pb(Zr,Ti)O3 (PZT), barium titanate (BaTiO3), and lithium niobate (LiNbO3), and piezoelectric polymers such as poly (vinylidene fluoride) (PVDF) and its copolymers. PZT has been widely studied for its superior piezo-photocatalytic activity, owing to its high piezoelectric response and semiconducting properties.
Li et al. found the responses of BiOCl to both ultrasonic and light irradiation and introduced the piezo-photocatalysis characteristics of BiOCl [90]. The study presented a facile hydrothermal method for synthesizing BiOCl nanosheets with highly exposed (001) or (101) crystal facets by simply tuning the nitrate acid addition, indicating that facet engineering can alter the material activity under different irradiation conditions. BiOCl with a higher degree of (001) plane exposure exhibited greater activity than that with more (101) plane exposure owing to differences in their band structures, active site densities, and charge separation efficiencies. Their responses to light and vibration are demonstrated in Figure 7, as Ag deposition indicates active sites during the reaction. The top facet was 001 and the side facet was 101. The response to light irradiation occurred on the 001 facet in BiOCl (001 dominant), since the response to ultrasonic activity was initiated on the 101 facet, as shown in Figure 7a–f. Under identical conditions, it was found that BiOCl (001 dominant) exhibited a surface-selective charge separation behavior under photoinduced conditions, a phenomenon that was also observed in other layered bismuth-based photocatalysts, such as Bi2WO6 [94] and BiOBr [6]. Figure 7g–l shows the Ag deposition of BiOCl (101 dominant) under the same conditions, indicating a higher density of reactive sites than for 001 dominant BiOCl due to its higher plane thickness. For BiOCl with both facet types, a synergistic effect of light and vibration irradiation was observed in enhanced Ag reduction, demonstrating the synergistic effect of piezo materials and photocatalysis. Taken together, these findings deepen the understanding of how crystal plane regulation affects planar charge separation and active sites in piezoelectric-photocoupled catalytic reactions. Despite the challenges of controlling the synthesis of these highly active crystal planes, the results of these studies provide a promising avenue for the development of more efficient and effective catalysts.
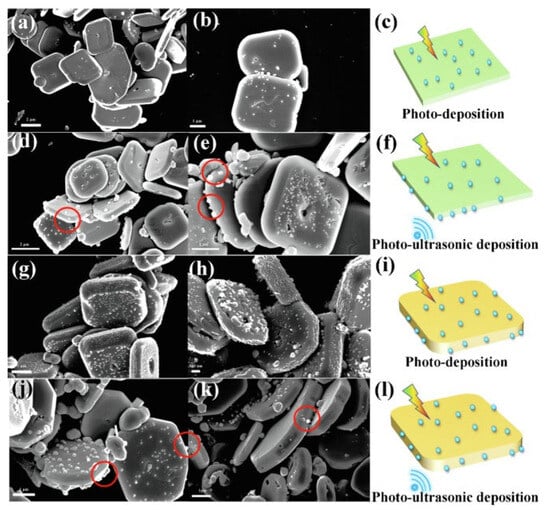
Figure 7. SEM images of BiOCl (001 dominant) with Ag reduction under light (a,b) and photo-piezo conditions (d,e), and schematic illustrations (c,f), respectively. SEM images of BiOCl (001 dominant) with Ag reduction under light (g,h) and photo-piezo conditions (j,k), and schematic illustrations (i,l), respectively [90].
The work by Singh et al. highlights the enhancement of piezoelectric-NaNbO3 nanostructures in both photocatalytic and photoelectrochemical activity using the piezo-phototronic effect [92]. Specifically, NaNbO3 under ultrasonic conditions boosted the photocatalytic degradation of methylene blue organic dye around 115%. The enhancement was initiated by the bias potential introduced by the piezo characteristic of NaNbO3, which subsequently hindered the recombination of the photogenerated charge carriers and increased the band alignment at the NaNbO3/electrolyte interface. Figure 8a presents NaNbO3′s band edge alignment at the interface with the electrolyte. When piezo potential is presented, the material rods push electrons and holes to the interface of the material and electrolyte and lifts the valence band to increase the oxidation potential. Similar band-bending models were proposed by Banoo et al. [95] and Li et al. [96] in studying Bi4TaO8Cl and BiOCl/NaNbO3, respectively. Banoo et al. studied the effect of the piezo nature on the generation of ROS. Bi4TaO8Cl is not able to produce •O2− in a photocatalytic process, as conduction is positioned at −0.30 eV, higher than the O2/•O2− potential of −0.33 eV. Under ultrasonication, the piezo effect triggers band bending that negatively shifts the conduction band to beyond −0.33 eV, allowing the reaction of photocatalytically generated electrons for •O2− production [95]. The same results were obtained by Li et al., in that piezo-triggered band bending initiated not only the single-component material but also the composite, e.g., BiOCl/NaNbO3 [96]. These works with piezo-photocatalysts in both single material and composite form demonstrated the synergy of the piezo effect and enhanced photocatalysis in two ways. One is bending the band structure for a higher capacity of the redox reaction, and the other is contributing to the charger transfer for more efficient transformation of electrons and holes.
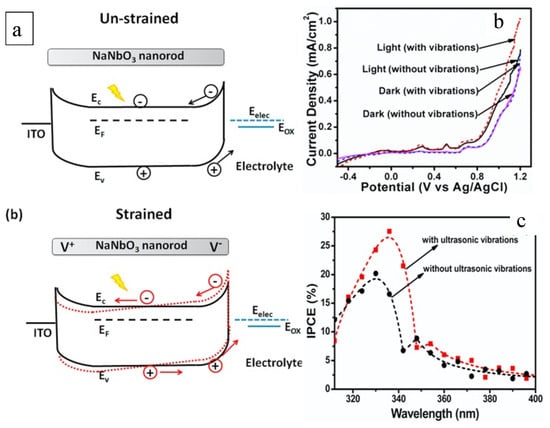
Figure 8. (a) Band structure of NaNbO3 nanorods with and without the peizo effect under straining conditions; (b) Current–potential curves of NaNbO3 nanorods under different light and vibration conditions; (c) Incident photon to current conversion efficiency (IPCE) of NaNbO3 photoanodes [92].
The piezo effect initiated by vibration allows the activity of NaNbO3 in the dark and enhances its performance in the light (Figure 8b), leading to a higher conversion efficiency of incident photons (Figure 8c). Yu et al. doped BaTiO3 with La and Li and obtained 4.6- and 3.9-times higher performance in removing dye pollutants compared with the pure BaTiO3 [93]. The significant improvement was attributed to the boosted separation of photocatalytically generated electrons and holes by the piezo built-in bias potential. By carefully inducing electron donors and acceptors using the doping method, the band structure was modified for enhanced promotion of the separation of charge carriers by the piezo effect. The doped piezo BaTiO3 exhibited higher activity in degrading anionic methyl blue and malachite green dyes, showing kinetic constants of 0.067 and 1.379 min−1, respectively. Since the constants were 0.274 and 0.029 min−1 for the cationic dyes Rhodamine B and methyl orange, respectively, this study offered a piezo-photocatalyst design for particularly charged pollutants.
The latter category is a hybrid system with the combination of piezo material and photocatalysis, for instance BiOCl/NaNbO3, KNbO3/ZnO, ZnO/PVDF, TiO2/PZT, ZnTe/CdSe, etc., of which the built-in electric field promotes the charge migration. In Li et al.’s work [97], KNbO3/ZnO nanocomposite was studied for piezo/photocatalytic degradation of methyl orange under simulated sunlight and ultrasonic vibration. The study demonstrated the enhanced catalytic performance of the KNbO3/ZnO composite compared to ZnO alone, both in terms of photocatalysis and piezocatalysis, exhibiting a 2.47 times higher photocatalysis rate than that of ZnO. The redistribution of piezo-photoinduced charge carriers in the component was attributed to an electric potential field formed at the material interface. With its favorable catalytic activity, stability, and ability to harness solar and mechanical energy, KNbO3 holds promising potential for boosting piezo-photocatalysis. One advantage of the hybrid system is that the piezo potential can cause band bending of the photocatalysts. ROS are the most significant factor in the catalytic process in organic pollutant removal. When the structures of the valence and conduction bands fit the redox potential of −0.33 V for •O2−/O2 and +1.9 V for •OH/OH− vs. NHE, ROS are generated from the reactions of electrons and holes from the band with O2 and OH− after moving to the catalyst surface for degradation. However, necessary strategies are required to fit the photocatalyst band structure to a suitable position for the redox reaction. Band bending offered by piezo potential allows the initiation of photocatalysis without modification. As shown in Figure 9, in the piezo-photocatalyst system, when the piezo effect occurs, the band structure of ZnTe bends, thus fitting both the reduction and oxidation positions as well as the alignment of the band position to make charge transfer feasible [98]. The unimpeded channel of charge migration results in enhanced conversion of light and mechanical energy and, at the same time, improved reaction performance.
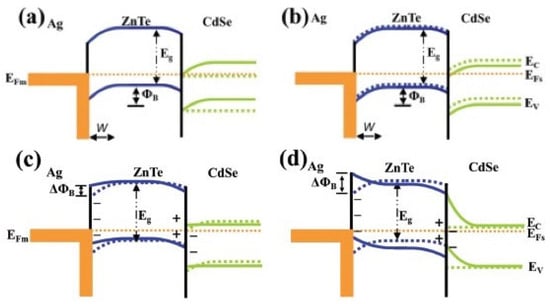
Figure 9. Illustration of band alignment and bending of the Ag/ZnTe/CdSe composite (a) in the ambient environment; (b) under light irradiation; (c) under a compressive load and light irradiation; (d) under a > 0.25 kgf compressive load [98].
The degradation efficiency is affected by the charge transfer between piezo- and photomaterials, so engineering the characteristics of the interface of piezo-photocatalyst components is also an important research point. An atomic layer forms as the interface in the synthetic process and is usually explored by studying the band structure modification. The layer formation and interface characteristics can be analyzed by DFT, since the characterization methods are still insufficient. Liu et al. [99] studied the interface of BaTiO3/TiO2 via DFT analysis. Figure 10a illustrates the atomic bonding structure of the interface. The charge density distribution of the interface (Figure 10b) demonstrates that, with the application of voltage on the interface, the piezo property of the component enhances the charge redistribution. Electron density variation mainly occurs on the oxygen atom of TiO2. This implies that there is a greater concentration of electrons gathered around the oxygen atoms, particularly those associated with TiO2. Consequently, there is an increased chance of electron interaction with O2, resulting in the production of a higher quantity of •O2− active radicals. This heightened electron availability and subsequent radical formation contribute to the enhancement of catalytic performance.
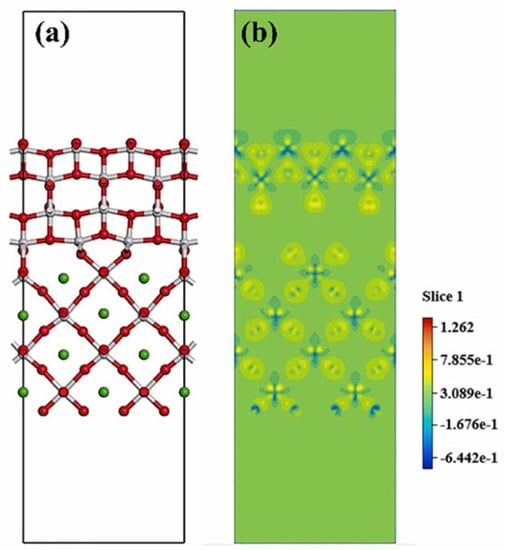
Figure 10. (a) Schematic DFT diagram of the interface structure of BaTiO3/TiO2; (b) Deformation charge density of atomic charge with piezoelectric potential [99].
ZnO nanowire is an electricity generator under vibration activation, showing a piezo characteristic [53]. As a piezocatalyst, ZnO was also studied for its photocatalytic performance [100]. To benefit the capacity of harvesting natural energy and providing sufficient reactive sites, flat-shaped ZnO was investigated for degrading hormones in wastewater under the activation of vibration and solar energies. The degradation of testosterone was limited under only sonication, indicating that the piezo reaction was insufficient in treating this organic pollutant in wastewater. However, the degradation performance was significantly improved under piezo-photocatalytic reaction conditions. A similar result was obtained in the degradation of β-estradiol, which is also considered to be an emerging contaminant. The improvement in efficiency was demonstrated in a scavenger study and a schematic representation is shown in Figure 11 [100]. In the piezo-catalytic process, the valence band is not activated, as the light irradiation trigger is absent, and, therefore, the hydroxyl radicals that mainly contribute to the testosterone degradation are not produced. Moreover, the inhibited generation of holes on the valence band by 2-propanol (IPOH) in the photocatalytic process significantly influences the degradation of both testosterone and β-estradiol, even with the simultaneous piezo reaction. These results demonstrated the efficient improvement in photocatalysis by the piezo process on the ZnO nanosheets (Figure 11a) via the generation of charge carriers and subsequent ROS generation. The piezo effect also modifies the band structure, triggering the photocatalytic reaction, as shown in Figure 11b, for a lean of valence and conduction bands that enhances the redox potential and results in higher degradation efficiency.
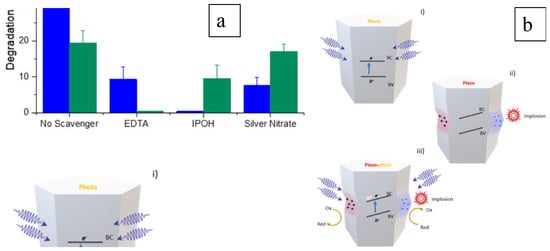
Figure 11. Reaction mechanism (a) studied by scavenger study and (b) represented schematically [100].
Regarding the hybrid system, structure is another crucial factor affecting the charge transfer and then performance. As the piezo reaction is activated by vibration, rod-shaped [101], nanowire [100,102], spherical [103], tetragonal nanocube [104], plate-form [105], and thin-film [106,107] materials have been developed to harvest mechanical energy from water flow and the environment. Alex et al. [105] developed a bilayered heterostructure with a BaTiO3 layer and MoO3 layer for piezoelectric and photocatalytic reactions, respectively. Under activation of mechanical vibration and light irradiation, the BaTiO3 layer was polarized to extract photocatalytically generated charge in the MoO3 layer, which was beneficial to electrons and holes in the redox reaction, simultaneously initiating degradation on the piezoelectric BaTiO3 layer.
Additionally, piezoelectric materials can also play a role in the conductive platform for electron transfer on the photocatalyst surface by supplying additional active sites. The composite photocatalytic and piezoelectric materials can synergistically promote their respective reaction activities. For instance, when Ag-modified NaNbO3 was used to prepare a piezoelectric photocatalyst composite, the rate of Rhodamine B degradation in wastewater was doubled under mechanical and light excitation conditions [20]. Similarly, Huang et al. [89] achieved 110% and 857% increases in hydrogen production efficiency in water compared to pure piezoelectric and photocatalytic materials, respectively, by preparing a composite piezoelectric photocatalytic material, PbTiO3/CdS. Preparing piezoelectric photocatalyst composites can better utilize the respective properties of photocatalysis and piezoelectric built-in electric fields and synergistically promote reaction activity.
When piezoelectric and photocatalytic materials form composite materials, the piezoelectric component generates a polarized electric field under continuous mechanical forces or deformation from external sources, which separates the photogenerated charge carriers produced by the photocatalytic component under light irradiation, capturing solar and mechanical energy and increasing the catalytic reaction efficiency. During the degradation of new pollutants, the migration and reaction of charges are affected by the piezoelectric–photocatalytic interaction, the distribution and changes in electric current and potential, the band tilt and transfer, and the adsorption and redox of surface free radicals, which continue to affect the migration and transfer mechanism of the piezoelectric built-in electric field charges and promote or inhibit catalytic reaction activity. The cooperative effect of piezoelectric coupling with photocatalysis, the effect and mechanism of the built-in electric field driving charge migration, and regulation and improvement of reaction activity also require further study.
Various strategies have been developed to modify piezo-photocatalysts in order to improve their performance, such as doping foreign elements [108], constructing heterojunctions [96,109], tuning microstructures (e.g., mesoporous TiO2) [110], designing assembly forms (e.g., nanofibers) [78], etc. These modifications can optimize the light absorption, charge transport, surface activity, mechanical stability, etc., of piezo-photocatalysts. Piezo-photocatalysis has shown promising results in practical applications, such as water treatment, hydrogen production, and antibacterial activity. However, there are still some challenges and limitations that need to be addressed, such as elucidation of the mechanism, the stability and recyclability of piezo-photocatalysts, and the scale-up of piezo-photocatalytic reactors. Therefore, further research and development are needed to explore the full potential of piezo-photocatalysis for environmental remediation and energy conversion. Piezo-photocatalysis has emerged as a promising technology in the field of environmental engineering. Combining photocatalysis and piezoelectricity, this technology can efficiently decompose organic pollutants and simultaneously generate clean energy. Research efforts have been made to explore the potential use of piezo-photocatalysis in various applications, such as wastewater treatment, air purification, and energy harvesting.
3.3. Application of Piezo-Photocatalysis for Organic Pollutant Removal
Piezo-photocatalysis has been applied for various environmental remediation purposes, such as degradation of organic pollutants (e.g., pharmaceuticals, pesticides), disinfection of pathogens (e.g., bacteria), hydrogen production from water splitting (e.g., using solar energy), etc. Piezo-photocatalysis has shown superior performance over conventional photocatalysis in terms of degradation rate and mineralization efficiency. Lan et al. [111] applied piezocatalyst BaTiO3 for the formation of ROS under ultrasonic vibration, with the activation of PMS, to degrade refractory benzothiazole in wastewater. Tian’s group deposited Ag on piezocatalyst BaTiO3 nanoparticles to increase the reaction performance of the dechlorination of 4-chlorophenol at a concentration 25 mg/L in wastewater [112]. Masekela et al. modified piezo material with fluorine-doped SnO2, forming F-SnO2/BaTiO3@SnO2 thin film to degrade dye pollutants and disinfect wastewater. Under ultrasonic excitation, the piezo composite generated an electric current as high as 1.3 mA and exhibited outstanding capacity for removing dyes and E. coli in wastewater [106]. Feng et al. combined piezocatalyst Pb(Zr,Ti)O3 and photocatalyst TiO2 in a core–shell structure for the purification of wastewater [113]. Removal of RhB, BPA, phenol, and p-chlorophenol in wastewater were effectively improved due to the introduction of the piezo effect. Yu et al. provided a useful idea for developing piezo-enhanced solar energy conversion [91]. They used KNbO3 nanosheets in PEC water splitting, reporting that the piezo effect and the sheet form of the material significantly enhanced the reaction performance, with a 55% increase in the photocurrent compared with the cube-form material.
Applying novel technology in practical wastewater treatment is challenging. Other than efforts aimed at improving treatment efficiency, strategies to lower functional cost and enhance material stability are required, such as imbedding reactive components in thin-film substrates that stabilize catalysts [114,115] on the bottom or suspend them in water flow and bulk foams [25] that allow catalysts to stay on top of the water for convenient collection. Regarding the enhancement of conversion of natural energy and the improvement of reaction efficiency, a higher number of reactive sites and a larger reaction surface are preferred. Compared to thin-film and bulk foam reaction systems, slurry systems provide more direct contact between the catalyst and pollutant and a larger surface for harvesting solar energy. However, the collection of particle materials after reaction for subsequent cycling of wastewater treatment is a huge challenge, since conventional retrieval methods such as filtration and precipitation are costly. Therefore, a convenient separation strategy of magnetic separation has been developed. It is a convenient method to retrieve catalysts from treated wastewater without significant material loss after efficient treatment [101,116,117]. Combination of a magnetic core and piezo-photocatalyst was applied to synthesize Fe3O4@SiO2@ZnO for wastewater treatment by Wu et al. [101]. ZnO nanorods were vertically grown on a magnetic sphere. SiO2 was applied to inhibit the charge transfer between Fe3O4 and the piezo-photocatalyst. Other than the enhanced reaction efficiency in the simultaneous piezo- and photoinduced situation, the composite had a superparamagnetism of 23.2 emu/g, which enabled good dispersion in the solution and convenient collection after several reaction cycles. This magnetic characteristic of micro- and nanoparticle materials provides prospects for wide application of piezo-photocatalysis for highly efficient wastewater treatment.
This entry is adapted from the peer-reviewed paper 10.3390/cryst13091382
This entry is offline, you can click here to edit this entry!
