Granulocytes (neutrophils, eosinophils, and basophils) are the most abundant circulating cells in the innate immune system. Circulating granulocytes, primarily neutrophils, can cross the endothelial barrier and activate various effector mechanisms to combat invasive pathogens. Eosinophils and basophils also play an important role in allergic reactions and antiparasitic defense. Granulocytes also regulate the immune response, wound healing, and tissue repair by releasing of various cytokines and lipid mediators. The effector mechanisms of granulocytes include the production of reactive oxygen species (ROS), degranulation, phagocytosis, and the formation of DNA-containing extracellular traps.
- granulocytes
- extracellular traps
- mitochondria
- mitochondria-targeted antioxidants
1. Introduction
2. The Role of Mitochondria in Extracellular Trap Formation
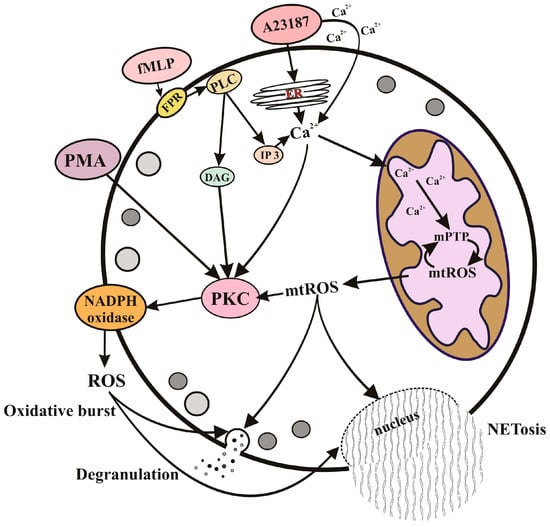
2.1. Neutrophil Extracellular Traps (NETs) and NETosis
2.2. The Mechanisms of Eosinophil and Basophil Extracellular Trap Formation
This entry is adapted from the peer-reviewed paper 10.3390/cells12182210
References
- References1. Karnovsky, M.L. The Metabolism of Leukocytes. Semin. Hematol. 1968, 5, 156–165.2. Furuno, T.; Ohyama, N.; Nakanishi, M. The Relation between Degranulation and Rapid Metabolic Responses in RBL-2H3 Cells. Biol. Pharm. Bull. 1999, 22, 310–312, doi:10.1248/bpb.22.310.3. Rodríguez-Espinosa, O.; Rojas-Espinosa, O.; Moreno-Altamirano, M.M.B.; López-Villegas, E.O.; Sánchez-García, F.J. Metabolic Requirements for Neutrophil Extracellular Traps Formation. Immunology 2015, 145, 213–224, doi:10.1111/imm.12437.4. Porter, L.; Toepfner, N.; Bashant, K.R.; Guck, J.; Ashcroft, M.; Farahi, N.; Chilvers, E.R. Metabolic Profiling of Human Eosinophils. Front. Immunol. 2018, 9, 1404, doi:10.3389/fimmu.2018.01404.5. Bao, Y.; Ledderose, C.; Seier, T.; Graf, A.F.; Brix, B.; Chong, E.; Junger, W.G. Mitochondria Regulate Neutrophil Activation by Generating ATP for Autocrine Purinergic Signaling. J. Biol. Chem. 2014, 289, 26794–26803, doi:10.1074/jbc.M114.572495.6. Sumbayev, V.V.; Nicholas, S.A.; Streatfield, C.L.; Gibbs, B.F. Involvement of Hypoxia-Inducible Factor-1 HiF(1alpha) in IgE-Mediated Primary Human Basophil Responses. Eur. J. Immunol. 2009, 39, 3511–3519, doi:10.1002/eji.200939370.7. Sharkia, I.; Erlich, T.H.; Landolina, N.; Assayag, M.; Motzik, A.; Rachmin, I.; Kay, G.; Porat, Z.; Tshori, S.; Berkman, N.; et al. Pyruvate Dehydrogenase Has a Major Role in Mast Cell Function, and Its Activity Is Regulated by Mitochondrial Microphthalmia Transcription Factor. Journal of Allergy and Clinical Immunology 2017, 140, 204–214.e8.8. Pavlyuchenkova, A.N.; Zinovkin, R.A.; Makievskaya, C.I.; Galkin, I.I.; Chelombitko, M.A. Mitochondria-Targeted Triphenylphosphonium-Based Compounds Inhibit FcεRI-Dependent Degranulation of Mast Cells by Preventing Mitochondrial Dysfunction through Erk1/2. Life Sci. 2022, 288, 120174, doi:10.1016/j.lfs.2021.120174.9. Korchak, H.M.; Rich, A.M.; Wilkenfeld, C.; Rutherford, L.E.; Weissmann, G. A Carbocyanine Dye, DiOC6(3), Acts as a Mitochondrial Probe in Human Neutrophils. Biochem. Biophys. Res. Commun. 1982, 108, 1495–1501, doi:10.1016/s0006-291x(82)80076-4.10. van Raam, B.J.; Sluiter, W.; de Wit, E.; Roos, D.; Verhoeven, A.J.; Kuijpers, T.W. Mitochondrial Membrane Potential in Human Neutrophils Is Maintained by Complex III Activity in the Absence of Supercomplex Organisation. PLoS One 2008, 3, e2013, doi:10.1371/journal.pone.0002013.11. Zheng, X.; Chen, M.; Meng, X.; Chu, X.; Cai, C.; Zou, F. Phosphorylation of Dynamin-Related Protein 1 at Ser616 Regulates Mitochondrial Fission and Is Involved in Mitochondrial Calcium Uniporter-Mediated Neutrophil Polarization and Chemotaxis. Mol. Immunol. 2017, 87, 23–32, doi:10.1016/j.molimm.2017.03.019.12. Bonjour, K.; Palazzi, C.; Silva, T.P.; Malta, K.K.; Neves, V.H.; Oliveira-Barros, E.G.; Neves, I.; Kersten, V.A.; Fortuna, B.T.; Samarasinghe, A.E.; et al. Mitochondrial Population in Mouse Eosinophils: Ultrastructural Dynamics in Cell Differentiation and Inflammatory Diseases. Front Cell Dev Biol 2022, 10, 836755, doi:10.3389/fcell.2022.836755.13. Zhang, B.; Alysandratos, K.-D.; Angelidou, A.; Asadi, S.; Sismanopoulos, N.; Delivanis, D.-A.; Weng, Z.; Miniati, A.; Vasiadi, M.; Katsarou-Katsari, A.; et al. Human Mast Cell Degranulation and Preformed TNF Secretion Require Mitochondrial Translocation to Exocytosis Sites: Relevance to Atopic Dermatitis. J. Allergy Clin. Immunol. 2011, 127, 1522–1531.e8, doi:10.1016/j.jaci.2011.02.005.14. Grivennikova, V.G.; Vinogradov, A.D. Mitochondrial Production of Reactive Oxygen Species. Biochemistry 2013, 78, 1490–1511, doi:10.1134/S0006297913130087.15. Thomas, D.C. The Phagocyte Respiratory Burst: Historical Perspectives and Recent Advances. Immunol. Lett. 2017, 192, 88–96, doi:10.1016/j.imlet.2017.08.016.16. de Boer, M.; Roos, D. Metabolic Comparison between Basophils and Other Leukocytes from Human Blood. J. Immunol. 1986, 136, 3447–3454.17. Morshed, M.; Hlushchuk, R.; Simon, D.; Walls, A.F.; Obata-Ninomiya, K.; Karasuyama, H.; Djonov, V.; Eggel, A.; Kaufmann, T.; Simon, H.-U.; et al. NADPH Oxidase-Independent Formation of Extracellular DNA Traps by Basophils. J. Immunol. 2014, 192, 5314–5323, doi:10.4049/jimmunol.1303418.18. Petreccia, D.C.; Nauseef, W.M.; Clark, R.A. Respiratory Burst of Normal Human Eosinophils. J. Leukoc. Biol. 1987, 41, 283–288, doi:10.1002/jlb.41.4.283.19. Lacy, P.; Abdel-Latif, D.; Steward, M.; Musat-Marcu, S.; Man, S.F.P.; Moqbel, R. Divergence of Mechanisms Regulating Respiratory Burst in Blood and Sputum Eosinophils and Neutrophils from Atopic Subjects. J. Immunol. 2003, 170, 2670–2679, doi:10.4049/jimmunol.170.5.2670.20. LeSuer, W.E.; Kienzl, M.; Ochkur, S.I.; Schicho, R.; Doyle, A.D.; Wright, B.L.; Rank, M.A.; Krupnick, A.S.; Kita, H.; Jacobsen, E.A. Eosinophils Promote Effector Functions of Lung Group 2 Innate Lymphoid Cells in Allergic Airway Inflammation in Mice. J. Allergy Clin. Immunol. 2023, doi:10.1016/j.jaci.2023.03.023.21. Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-Derived Cytokines: Facts beyond Expression. Front. Immunol. 2014, 5, 508, doi:10.3389/fimmu.2014.00508.22. Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-Derived Suppressor Cells Coming of Age. Nat. Immunol. 2018, 19, 108–119, doi:10.1038/s41590-017-0022-x.23. Zhao, Y.; Rahmy, S.; Liu, Z.; Zhang, C.; Lu, X. Rational Targeting of Immunosuppressive Neutrophils in Cancer. Pharmacol. Ther. 2020, 212, 107556, doi:10.1016/j.pharmthera.2020.107556.24. Cheng, G.; Hardy, M.; Topchyan, P.; Zander, R.; Volberding, P.; Cui, W.; Kalyanaraman, B. Mitochondria-Targeted Hydroxyurea Inhibits OXPHOS and Induces Antiproliferative and Immunomodulatory Effects. iScience 2021, 24, 102673, doi:10.1016/j.isci.2021.102673.25. Miyake, K.; Shibata, S.; Yoshikawa, S.; Karasuyama, H. Basophils and Their Effector Molecules in Allergic Disorders. Allergy 2021, 76, 1693–1706, doi:10.1111/all.14662.26. Zakharova, V.V.; Pletjushkina, O.Y.; Galkin, I.I.; Zinovkin, R.A.; Chernyak, B.V.; Krysko, D.V.; Bachert, C.; Krysko, O.; Skulachev, V.P.; Popova, E.N. Low Concentration of Uncouplers of Oxidative Phosphorylation Decreases the TNF-Induced Endothelial Permeability and Lethality in Mice. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 968–977, doi:10.1016/j.bbadis.2017.01.024.27. Yamada, T.; Tani, Y.; Nakanishi, H.; Taguchi, R.; Arita, M.; Arai, H. Eosinophils Promote Resolution of Acute Peritonitis by Producing Proresolving Mediators in Mice. FASEB J. 2011, 25, 561–568, doi:10.1096/fj.10-170027.28. Ogawa, M.; Ishihara, T.; Isobe, Y.; Kato, T.; Kuba, K.; Imai, Y.; Uchino, Y.; Tsubota, K.; Arita, M. Eosinophils Promote Corneal Wound Healing via the 12/15-Lipoxygenase Pathway. FASEB J. 2020, 34, 12492–12501, doi:10.1096/fj.202000483R.29. Sud’ina, G.F.; Golenkina, E.A.; Prikhodko, A.S.; Kondratenko, N.D.; Gaponova, T.V.; Chernyak, B.V. Mitochondria-Targeted Antioxidant SkQ1 Inhibits Leukotriene Synthesis in Human Neutrophils. Front. Pharmacol. 2022, 13, 1023517, doi:10.3389/fphar.2022.1023517.30. Koenderman, L.; Tesselaar, K.; Vrisekoop, N. Human Neutrophil Kinetics: A Call to Revisit Old Evidence. Trends Immunol. 2022, 43, 868–876, doi:10.1016/j.it.2022.09.008.31. Park, Y.M.; Bochner, B.S. Eosinophil Survival and Apoptosis in Health and Disease. Allergy Asthma Immunol. Res. 2010, 2, 87–101, doi:10.4168/aair.2010.2.2.87.32. Ohnmacht, C.; Voehringer, D. Basophil Effector Function and Homeostasis during Helminth Infection. Blood 2009, 113, 2816–2825, doi:10.1182/blood-2008-05-154773.33. Wedi, B.; Straede, J.; Wieland, B.; Kapp, A. Eosinophil Apoptosis Is Mediated by Stimulators of Cellular Oxidative Metabolisms and Inhibited by Antioxidants: Involvement of a Thiol-Sensitive Redox Regulation in Eosinophil Cell Death. Blood, The Journal of the American Society of Hematology 1999, 94, 2365–2373.34. Ilmarinen, P.; Moilanen, E.; Kankaanranta, H. Mitochondria in the Center of Human Eosinophil Apoptosis and Survival. Int. J. Mol. Sci. 2014, 15, 3952–3969, doi:10.3390/ijms15033952.35. Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535, doi:10.1126/science.1092385.36. Steinberg, B.E.; Grinstein, S. Unconventional Roles of the NADPH Oxidase: Signaling, Ion Homeostasis, and Cell Death. Sci. STKE 2007, 2007, e11, doi:10.1126/stke.3792007pe11.37. Vorobjeva, N.V.; Pinegin, B.V. Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Health and Disease. Biochemistry 2014, 79, 1286–1296, doi:10.1134/S0006297914120025.38. Pinegin, B.; Vorobjeva, N.; Pinegin, V. Neutrophil Extracellular Traps and Their Role in the Development of Chronic Inflammation and Autoimmunity. Autoimmun. Rev. 2015, 14, 633–640, doi:10.1016/j.autrev.2015.03.002.39. Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2018, 18, 134–147, doi:10.1038/nri.2017.105.40. Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190, doi:10.1134/S0006297920100065.41. Vorobjeva, N.V. Neutrophil Extracellular Traps: New Aspects. Moscow Univ. Biol. Sci. Bull. 2020, 75, 173–188, doi:10.3103/S0096392520040112.42. Svistushkin, V.M.; Nikiforova, G.N.; Vorobjeva, N.V.; Dekhanov, A.S.; Dagil, Y.A.; Bredova, O.Y.; Eremeeva, K.V. [Neutrophil extracellular traps in the pathogenesis of chronic rhinosinusitis]. Vestn. Otorinolaringol. 2021, 86, 105–112, doi:10.17116/otorino202186061105.43. Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like Release of Mitochondrial DNA by Eosinophils Contributes to Antibacterial Defense. Nat. Med. 2008, 14, 949–953, doi:10.1038/nm.1855.44. von Köckritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-Independent Antimicrobial Activity of Mast Cells by Means of Extracellular Trap Formation. Blood 2008, 111, 3070–3080, doi:10.1182/blood-2007-07-104018.45. Ingelsson, B.; Söderberg, D.; Strid, T.; Söderberg, A.; Bergh, A.-C.; Loitto, V.; Lotfi, K.; Segelmark, M.; Spyrou, G.; Rosén, A. Lymphocytes Eject Interferogenic Mitochondrial DNA Webs in Response to CpG and Non-CpG Oligodeoxynucleotides of Class C. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, E478–E487, doi:10.1073/pnas.1711950115.46. Granger, V.; Faille, D.; Marani, V.; Noël, B.; Gallais, Y.; Szely, N.; Flament, H.; Pallardy, M.; Chollet-Martin, S.; de Chaisemartin, L. Human Blood Monocytes Are Able to Form Extracellular Traps. J. Leukoc. Biol. 2017, 102, 775–781, doi:10.1189/jlb.3MA0916-411R.47. Chow, O.A.; von Köckritz-Blickwede, M.; Bright, A.T.; Hensler, M.E.; Zinkernagel, A.S.; Cogen, A.L.; Gallo, R.L.; Monestier, M.; Wang, Y.; Glass, C.K.; et al. Statins Enhance Formation of Phagocyte Extracellular Traps. Cell Host Microbe 2010, 8, 445–454, doi:10.1016/j.chom.2010.10.005.48. Yipp, B.G.; Kubes, P. NETosis: How Vital Is It? Blood 2013, 122, 2784–2794, doi:10.1182/blood-2013-04-457671.49. Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable Neutrophils Release Mitochondrial DNA to Form Neutrophil Extracellular Traps. Cell Death Differ. 2009, 16, 1438–1444, doi:10.1038/cdd.2009.96.50. Li, T.; Zhang, Z.; Li, X.; Dong, G.; Zhang, M.; Xu, Z.; Yang, J. Neutrophil Extracellular Traps: Signaling Properties and Disease Relevance. Mediators Inflamm. 2020, 2020, 9254087, doi:10.1155/2020/9254087.51. Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A Myeloperoxidase-Containing Complex Regulates Neutrophil Elastase Release and Actin Dynamics during NETosis. Cell Rep. 2014, 8, 883–896, doi:10.1016/j.celrep.2014.06.044.52. Gray, R.D.; Lucas, C.D.; MacKellar, A.; Li, F.; Hiersemenzel, K.; Haslett, C.; Davidson, D.J.; Rossi, A.G. Activation of Conventional Protein Kinase C (PKC) Is Critical in the Generation of Human Neutrophil Extracellular Traps. J. Inflamm. 2013, 10, 12, doi:10.1186/1476-9255-10-12.53. Vorobjeva, N.V.; Vakhlyarskaya, S.S.; Chernyak, B.V. The Role of Protein Kinase C Isoforms in the Formation of Neutrophil Extracellular Traps. Moscow Univ. Biol. Sci. Bull. 2022, 77, 112–118, doi:10.3103/S0096392522020122.54. Vorobjeva, N.; Dagil, Y.; Pashenkov, M.; Pinegin, B.; Chernyak, B. Protein Kinase C Isoforms Mediate the Formation of Neutrophil Extracellular Traps. Int. Immunopharmacol. 2023, 114, 109448, doi:10.1016/j.intimp.2022.109448.55. Amulic, B.; Knackstedt, S.L.; Abu Abed, U.; Deigendesch, N.; Harbort, C.J.; Caffrey, B.E.; Brinkmann, V.; Heppner, F.L.; Hinds, P.W.; Zychlinsky, A. Cell-Cycle Proteins Control Production of Neutrophil Extracellular Traps. Dev. Cell 2017, 43, 449–462.e5, doi:10.1016/j.devcel.2017.10.013.56. Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK Pathway Is Required for Neutrophil Extracellular Trap Formation. Nat. Chem. Biol. 2011, 7, 75–77, doi:10.1038/nchembio.496.57. Vorobjeva, N.V. Participation of Non-Receptor Src-Family Tyrosine Kinases in the Formation of Neutrophil Extracellular Traps. Moscow Univ. Biol. Sci. Bull. 2023, 78, 8–13, doi:10.3103/S0096392523010078.58. Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus Aureus. J. Immunol. 2010, 185, 7413–7425, doi:10.4049/jimmunol.1000675.59. Parker, H.; Dragunow, M.; Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Requirements for NADPH Oxidase and Myeloperoxidase in Neutrophil Extracellular Trap Formation Differ Depending on the Stimulus. J. Leukoc. Biol. 2012, 92, 841–849, doi:10.1189/jlb.1211601.60. Arai, Y.; Nishinaka, Y.; Arai, T.; Morita, M.; Mizugishi, K.; Adachi, S.; Takaori-Kondo, A.; Watanabe, T.; Yamashita, K. Uric Acid Induces NADPH Oxidase-Independent Neutrophil Extracellular Trap Formation. Biochem. Biophys. Res. Commun. 2014, 443, 556–561, doi:10.1016/j.bbrc.2013.12.007.61. Pieterse, E.; Rother, N.; Yanginlar, C.; Gerretsen, J.; Boeltz, S.; Munoz, L.E.; Herrmann, M.; Pickkers, P.; Hilbrands, L.B.; van der Vlag, J. Cleaved N-Terminal Histone Tails Distinguish between NADPH Oxidase (NOX)-Dependent and NOX-Independent Pathways of Neutrophil Extracellular Trap Formation. Ann. Rheum. Dis. 2018, 77, 1790–1798, doi:10.1136/annrheumdis-2018-213223.62. de Bont, C.M.; Boelens, W.C.; Pruijn, G.J.M. NETosis, Complement, and Coagulation: A Triangular Relationship. Cell. Mol. Immunol. 2019, 16, 19–27, doi:10.1038/s41423-018-0024-0.63. Tatsiy, O.; McDonald, P.P. Physiological Stimuli Induce PAD4-Dependent, ROS-Independent NETosis, With Early and Late Events Controlled by Discrete Signaling Pathways. Front. Immunol. 2018, 9, 2036, doi:10.3389/fimmu.2018.02036.64. Chen, K.; Nishi, H.; Travers, R.; Tsuboi, N.; Martinod, K.; Wagner, D.D.; Stan, R.; Croce, K.; Mayadas, T.N. Endocytosis of Soluble Immune Complexes Leads to Their Clearance by FcγRIIIB but Induces Neutrophil Extracellular Traps via FcγRIIA in Vivo. Blood 2012, 120, 4421–4431, doi:10.1182/blood-2011-12-401133.65. Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H. von; Zychlinsky, A. Diverse Stimuli Engage Different Neutrophil Extracellular Trap Pathways. Elife 2017, 6, doi:10.7554/eLife.24437.66. Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil Extracellular Traps Enriched in Oxidized Mitochondrial DNA Are Interferogenic and Contribute to Lupus-like Disease. Nat. Med. 2016, 22, 146–153, doi:10.1038/nm.4027.67. Vorobjeva, N.; Galkin, I.; Pletjushkina, O.; Golyshev, S.; Zinovkin, R.; Prikhodko, A.; Pinegin, V.; Kondratenko, I.; Pinegin, B.; Chernyak, B. Mitochondrial Permeability Transition Pore Is Involved in Oxidative Burst and NETosis of Human Neutrophils. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165664, doi:10.1016/j.bbadis.2020.165664.68. Vorobjeva, N.V.; Chernyak, B.V. NADPH Oxidase Modulates Ca2+-Dependent Formation of Neutrophil Extracellular Traps. Moscow Univ. Biol. Sci. Bull. 2020, 75, 104–109, doi:10.3103/S0096392520030104.69. Bernardi, P.; Rasola, A.; Forte, M.; Lippe, G. The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol. Rev. 2015, 95, 1111–1155, doi:10.1152/physrev.00001.2015.70. Dumas, J.F.; Argaud, L.; Cottet-Rousselle, C.; Vial, G.; Gonzalez, C.; Detaille, D.; Leverve, X.; Fontaine, E. Effect of Transient and Permanent Permeability Transition Pore Opening on NAD(P)H Localization in Intact Cells. J. Biol. Chem. 2009, 284, 15117–15125, doi:10.1074/jbc.M900926200.71. Dunham-Snary, K.J.; Surewaard, B.G.; Mewburn, J.D.; Bentley, R.E.; Martin, A.Y.; Jones, O.; Al-Qazazi, R.; Lima, P.A.; Kubes, P.; Archer, S.L. Mitochondria in Human Neutrophils Mediate Killing of Staphylococcus Aureus. Redox Biol 2022, 49, 102225, doi:10.1016/j.redox.2021.102225.72. Ueki, S.; Melo, R.C.N.; Ghiran, I.; Spencer, L.A.; Dvorak, A.M.; Weller, P.F. Eosinophil Extracellular DNA Trap Cell Death Mediates Lytic Release of Free Secretion-Competent Eosinophil Granules in Humans. Blood 2013, 121, 2074–2083, doi:10.1182/blood-2012-05-432088.73. Germic, N.; Fettrelet, T.; Stojkov, D.; Hosseini, A.; Horn, M.P.; Karaulov, A.; Simon, D.; Yousefi, S.; Simon, H.-U. The Release Kinetics of Eosinophil Peroxidase and Mitochondrial DNA Is Different in Association with Eosinophil Extracellular Trap Formation. Cells 2021, 10, doi:10.3390/cells10020306.74. Germic, N.; Stojkov, D.; Oberson, K.; Yousefi, S.; Simon, H.-U. Neither Eosinophils nor Neutrophils Require ATG5-Dependent Autophagy for Extracellular DNA Trap Formation. Immunology 2017, 152, 517–525, doi:10.1111/imm.12790.
