Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Osteoporosis is a common skeletal disorder which can severely limit one’s ability to complete daily tasks due to the increased risk of bone fractures, reducing quality of life. Spinal cord injury (SCI) can also result in osteoporosis and sarcopenia.
- neurodegeneration
- osteoporosis
- spinal cord injury
1. Introduction
Spinal cord injury (SCI) is a severe neurological disorder that results from sudden and damaging impact to the spine and vertebrae [1][2]. SCI is one of the most commonly caused damages in vehicle injuries [3], but can also be caused by falls, athletic injuries, and various other reasons [4]. SCI impacts more than 10,000 individuals each year and poses a significant economic burden to the U.S [5]. SCI can be detrimental and life threatening, and while there are therapeutic modalities being studied, more research on how to mitigate the short- and long-term effects of SCI is still needed. The immediate impacts of SCI can vary and depend largely on the specific location and magnitude of the injury [1][6]. In general, the higher up the level of injury is to the spinal cord, the more severe the symptoms. Injuries to the spinal cord of any magnitude and location can have both localized and global effects on bone composition. The local effects include paralysis, reduced function in the lower body, and bone loss, most commonly in the femurs, tibias, fibulas, and pelvic bones. The global effects of SCI (i.e., neurogenic bone loss) include changes in neural signaling over time, which can lead to a disruption in bone remodeling throughout the body, not just in regions directly impacted by the SCI. The global effects of SCI may also include disruptions to bone vascularity, as there is a synergistic relationship between the skeletal and vascular systems. A decrease in bone vascularity and reduced neoangiogenesis can limit the healing capacity and progress of SCI rehabilitation modalities, and thus limit bone remodeling and repair [7]. People with a SCI are two to five times more likely to die prematurely than people without an SCI, and this carries substantial individual and societal costs. Short-term impacts often include gliosis, axonal damage, neuronal death, immobilization, and a loss of sensory and motor function, while long-term impacts include organ dysfunction, sarcopenia, osteopenia, bone fractures, and osteoporosis [1][4][8].
Demyelination and axonal degeneration are short-term but chronic outcomes of SCI, because they last for prolonged periods of time after the injury and are often irreversible [2][6]. Axonal degeneration occurs when the axons are lesioned, causing severe neuronal transmission deficits distal to the lesion site. This damage is furthered if the axon is lesioned in the central nervous system (CNS). Although there are potential therapeutic approaches to slowing axonal degeneration, this damage is usually permanent if the axonal lesion site is in the CNS [1][4]. Demyelination and a buildup of myelin debris are other immediate outcomes of SCI, which then lead to excessive levels of gliosis and glial scar formation [9][10]. These are just some of the immediate, short-term effects of SCI that come along with a multitude of long-term effects.
Many of the long-term outcomes of SCI are related to muscle and bone loss due to immobilization. Due to lack of physical activity and increased immobilization after one suffers from severe SCI, muscle and bone tissue severely decrease [11][12]. Osteoporosis is a common issue experienced after SCI and is defined as a skeletal disorder in which bone strength is compromised, leaving a person with a greater risk of fracture [13][14]. Individuals with osteoporosis experience large levels of osteopenia and are prone to fractures, which severely decrease quality of life and require substantial medical resources. Due to osteopenia after SCI, bone fractures are extremely common in individuals with SCI, because of their lower osteogenic load and increased bone demineralization [15][16][17]. The absolute causes of bone loss after SCI are not yet known; however, some of the possible causes are neurogenic factors, hormonal factors, and sarcopenia [15][18]. Immobility and disuse are other causes of osteopenia and sarcopenia in SCI patients due to the decrease in mechanical loading in the bone while one recovers from SCI. Sarcopenia, also known as muscle loss, has been linked to being a possible cause of osteopenia; however, more research is needed to evaluate the relationship between osteopenia and sarcopenia in SCI [11][19]. Diagnosis, prevention, and treatment for decreasing osteopenia and osteoporosis after SCI are critical to helping the thousands of individuals who suffer from SCI each year [15][17].
Therapies for reducing the negative outcomes of SCI are urgently needed. Although there has been promising research on therapies such as blocking 4-1BB and RANKL signaling [20][21], increasing Wnt signaling and calcium-regulated hormones [22][23], and loading of the bones and muscles [24], further research is still needed and there is research being conducted now on prospects for SCI treatments.
2. Pathophysiology of Bone Loss after SCI
Individuals with complete paralysis after SCI show the most extensive bone loss and fracture risk [25][26]. Understanding the mechanisms that lead to bone loss and osteoporosis after SCI is important to determining how to slow bone loss after SCI. Common causes of bone loss after severe SCI are immobility and de-loading, which result in increased bone resorption and a decrease in osteoblast activity [4][27]. When one is immobile due to an injury, less stress is placed on the bones, leading to a direct response from other systems in the body, including the neurogenic and musculoskeletal systems [27]. Immobility has a direct effect on the musculoskeletal system, since it causes an increase in bone resorption and a decrease in osteoblast activity, resulting in osteopenia [4][27]. However, bone loss following SCI is believed to be distinct, as compared to the response to other disuse conditions in terms of both severity and mechanism. Although the focus is SCI, other factors secondary to SCI may also promote bone loss, including systemic hormonal changes, altered bone innervation, and impaired bone perfusion [26][28]. In an SCI study conducted on rats, significant bone loss was observed during a bone compartment analysis on the SCI animals compared to controls [11][29]. Overall, decreases in bone mineral content, trabecular structure, and bone mineral density were observed in all the SCI groups.
The next systems that immobilization and bone loss impact are the CNS, peripheral nervous system (PNS), and endocrine system. Bone cells have many nerve endings close to them, which greatly impact the CNS and PNS. Bone cells also connect the skeleton to the endocrine system through various receptors and neuromediators [27]. Skeletal loss may also promote sarcopenia and endocrine system dysfunction via multiple receptors and neuromediators, thus influencing the adipose tissue production of leptin and anorexigenics, which both affect bone remodeling [27][30]. Moreover, immobilization impacts skeletal vascularization, which is required for bone remodeling and osteoblast function. The resulting vasoconstriction further contributes to the muscular, endocrine, and nervous system impairments associated with osteoporosis in SCI patients.
The vascular system is a necessary contributor to osteogenesis after SCI. Neo-angiogenesis (i.e., the formation of new blood vessels) plays a crucial role in bone development after SCI, because it ensures that bone tissues are obtaining the necessary blood and oxygen supply to stimulate bone formation, maintenance, and repair [7]. Following SCI, individuals often experience disruptions to the circulatory system from mechanical trauma. Ischemia, hypoxia, and localized edema are potential secondary effects of SCI impacting the vascular system, thus impeding healing and rehabilitation [31]. The secondary effects of SCI on the vascular network not only potentially cause secondary injury and can further deteriorate bone and spinal cord tissue, but a reduced vascularity can also mitigate healing from SCI treatment [7][31]. Various SCI treatments, including cell transplantation, are ineffective if the local blood vessels are damaged, leading to a lack of oxygen and nutrients that the transplanted cells need for survival [31]. Pericytes and endothelial cells are important structures of the vascular system that play essential roles in angiogenesis; however, they cannot sustain and mediate angiogenesis to osteogenesis when there is damage to the blood vessels in the affected area [7][31]. Physical rehabilitation and therapeutic strategies, such as surgical anastomosis and exogenous pericyte cell transplantation, are available to help to stimulate angiogenesis after SCI [7]. Research is still limited on the effectiveness of therapy and rehabilitation for stimulating angiogenesis after SCI.
3. Therapeutic Strategies for Neurogenic Bone Loss after SCI
3.1. Pharmacological Therapy
Pharmacological therapies for the bone loss in SCI individuals have been relatively ineffective. While vitamin D supplementation is commonly used to restore the vitamin D levels in SCI individuals with a vitamin D deficiency, it has not been effective in preventing and restoring bone loss [32]. Thus, multiple pharmacological strategies may provide benefits for neurogenic bone loss after SCI. For example, ellagic acid (EA) has been found to bind to RANKL and downregulate osteoclast activity, although this endogenous compound may produce negative side effects at elevated concentrations [33][34][35]. Bisphosphonates and Denosumab have also been evaluated for their prevention of the loss of bone mass after SCI (Figure 1). Bisphosphonates act to slow bone loss by inhibiting bone resorption; these include Etidronate, Clodronate, Pamidronate, Tiludronate, and Alendronate [36][37][38]. Bisphosphonates used in SCI patients have been shown to reduce the risk of hip fractures (but not knee fractures) [29][39].
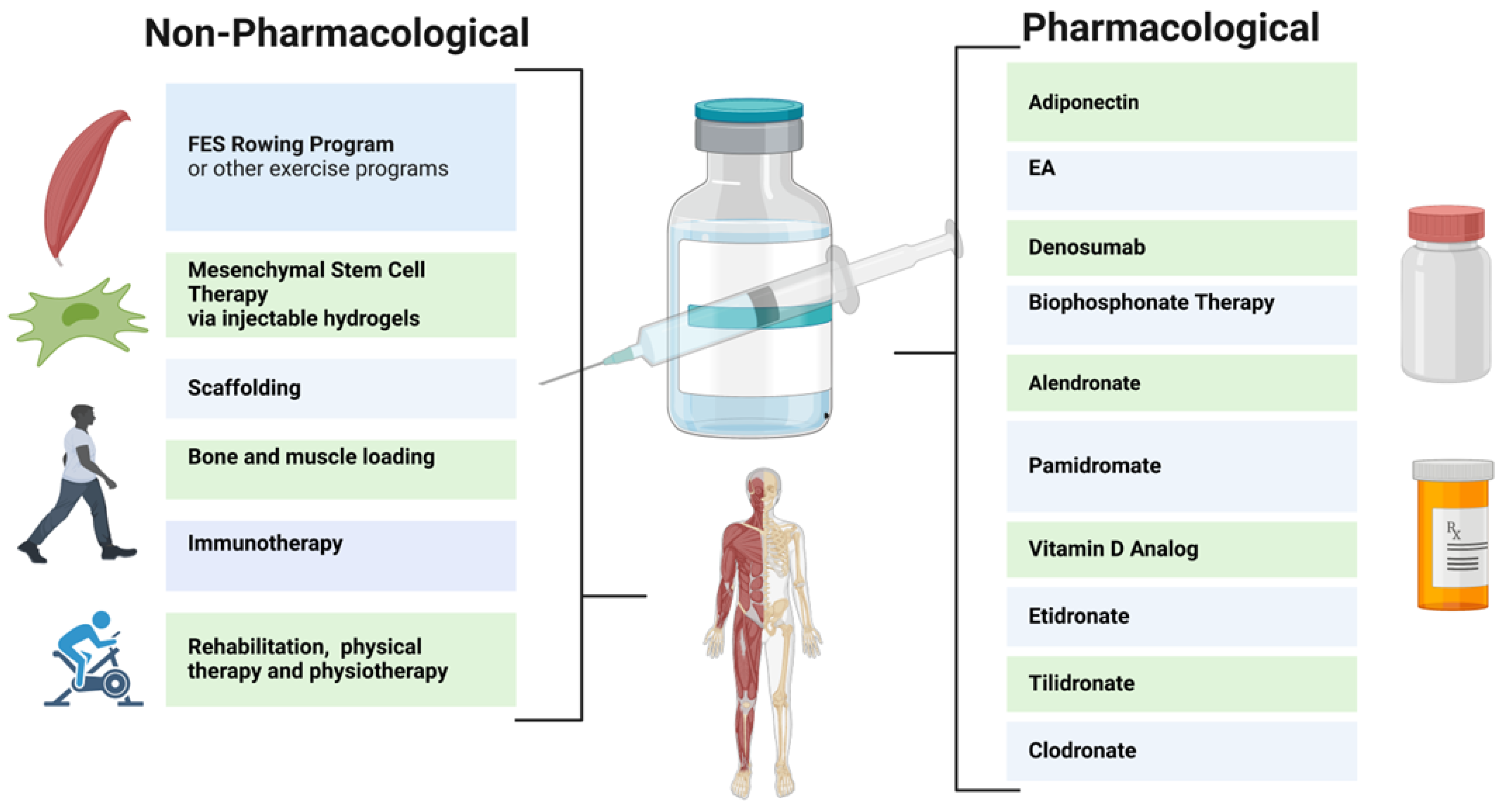
Figure 1. Rehabilitation Methods for Neurogenic Bone Loss After SCI.
Despite some success, the effects of bisphosphonates have been inconsistent. Clodronate, Etidronate, and Tiludronate have been shown to yield increased bone mass in less than one year post injury (Figure 1), whereas Alendronate improved bone mass in more than one year after injury [36]. However, Pamidronate was not shown to improve bone mass in this study. In addition, the prolonged use of bisphosphonate therapy may produce adverse effects such as osteonecrosis of the jaw; thus, judicial administration is advised [40]. These therapies are currently available in oral or intravenous administrations, and single annualendroal bisphosphonate injections may be available for SCI patients in the future [29][40][41]. In a recent larger clinical trial on patients with chronic SCI, Teriparatide treatment was used, which resulted in a significant increase in spine BMD at 1 year and further improvements in the hip at 2 years [42][43]. Furthermore, Denosumab, a monoclonal antibody to RANKL, is FDA approved for osteoporosis treatment [44][45]. Denosumab prevents bone loss in SCI patients via the inhibition of osteoclast activity via the RANKL pathway, however, it must be frequently administered [39][46][47][48]. Denosumab thus reduces bone resorption and increases bone mineral density, reducing the risk of fractures.
3.2. Nonpharmacological Therapy
Pharmacological therapies to date are limited, as they do not provide a significant restoration of damaged spinal cord parenchyma. Therefore, non-pharmacological approaches, such as mesenchymal stem cell (MSC) therapy, physiotherapy, immunotherapy, injectable hydrogels, and stem cell secretome therapy, are under consideration [49][50]. MSCs from the bone marrow, umbilical cord, and/or adipose tissue may reduce inflammation and provide neuroprotective effects to prevent further injury to the spinal cord near the impact site. Injectable hydrogels, which facilitate MSC targeting, are also being studied [51].
These therapies, in conjunction with weight-bearing rehabilitation, may be increasingly employed to decrease osteopenia in patients with SCI [52][53]. Following SCI, a primary catalyst behind bone loss is the decrease in mechanical loading. When individuals with SCI cease weight-bearing activities, they face a heightened susceptibility to rapid bone resorption and osteocyte apoptosis, frequently leading to the development of osteoporosis. Engaging in any form of mechanical loading on the skeletal system, including compression, tension, torsion, or bending, will uphold bone density and promote bone mass recovery [54]. Therapies aimed at this axial loading encompass activities such as walking, jogging, and jumping. Rehabilitations that stimulate mechanical loading are practical, non-invasive, and economical methods for stimulating bone regeneration [55]. Rehabilitation improves mechanical loading by exposing tissues to a range of strains and forces, prompting osteocytes to sense stress and begin to stimulate regeneration [56]. Reciprocally, the subjection of mechanical loading on tissues from rehabilitation has been shown to be an effective therapy for tissue regeneration, which ultimately improves the bone’s capacity for mechanical loading [55]. Rehabilitation also enhances mechanical loading by modifying and improving vascularization, thereby facilitating bone growth. Therapies with an increased musculoskeletal load have proven effective; however, this approach is limited in patients who are wheelchair-bound after SCI. Stand-up wheelchairs, standing frames, and suspended treadmills can provide useful alternatives [11][57][58]. Physical activity, which inherently stimulates the axial loading of the tibia, femur, and axial skeleton, may also promote bone density after SCI by improving bone vascularization and osteoblast activity [4][58].
Static loading and prone position muscle stimulation appear to be less effective techniques for the attenuation of bone loss after SCI [4][59]. Thus, functional electrical stimulation (FES) rowing following SCI has been evaluated. FES rowing employs cyclical exercise patterns coupled with electrical stimulation to simulate the functional motor patterns otherwise impaired by SCI. Rowing allows for paraparetic SCI patients to exercise in a sitting position (in some cases with a cycle ergometer), coordinating their upper body movements with the electrical stimulation of the lower body muscle groups to recreate the effects of full-body exercise [36][59][60]. In one trial, the bone loss in the distal femur and tibia appeared to be reduced in the majority of participants after 30 sessions; however, other results have suggested that bone loss is ameliorated with muscle electrical stimulation alone. Non-mechanical load-bearing exercises such as swimming and cycling are weaker therapies in terms of reducing bone loss; however, they have still been shown to be effective at maintaining muscle mass, which can indirectly reduce fracture risk. Further studies are therefore needed to determine how these therapies can be best implemented for SCI individuals who are wheelchair-bound. It is important to note that the extent of improvement in mechanical loading after SCI is highly dependent on individual aspects such as the severity of the injury. To yield the best results, rehabilitation should be started early, be consistent, and be tailored to individual needs and goals.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11092581
References
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019, 10, 282.
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018.
- Chen, Y.; Tang, Y.; Vogel, L.; DeVivo, M. Causes of spinal cord injury. Top. Spinal Cord Inj. Rehabil. 2013, 19, 1–8.
- Shams, R.; Drasites, K.P.; Zaman, V.; Matzelle, D.; Shields, D.C.; Garner, D.P.; Sole, C.J.; Haque, A.; Banik, N.L. The Pathophysiology of Osteoporosis after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 3057.
- Chen, L.W.; Glinsky, J.V.; Islam, S.; Hossain, M.; Boswell-Ruys, C.L.; Kataria, C.; Redhead, J.; Xiong, Y.; Gollan, E.; Costa, P.D.; et al. The effects of 10,000 voluntary contractions over 8 weeks on the strength of very weak muscles in people with spinal cord injury: A randomised controlled trial. Spinal Cord 2020, 58, 857–864.
- Fouad, K.; Popovich, P.G.; Kopp, M.A.; Schwab, J.M. The neuroanatomical–functional paradox in spinal cord injury. Nat. Rev. Neurol. 2021, 17, 53–62.
- Zhu, S.; Bennett, S.; Kuek, V.; Xiang, C.; Xu, H.; Rosen, V.; Xu, J. Endothelial cells produce angiocrine factors to regulate bone and cartilage via versatile mechanisms. Theranostics 2020, 10, 5957–5965.
- Rodriguez, G.; Berri, M.; Lin, P.; Kamdar, N.; Mahmoudi, E.; Peterson, M.D. Musculoskeletal morbidity following spinal cord injury: A longitudinal cohort study of privately-insured beneficiaries. Bone 2021, 142, 115700.
- Wang, S.; Deng, J.; Fu, H.; Guo, Z.; Zhang, L.; Tang, P. Astrocytes directly clear myelin debris through endocytosis pathways and followed by excessive gliosis after spinal cord injury. Biochem. Biophys. Res. Commun. 2020, 525, 20–26.
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 78.
- Lin, C.-Y.; Androjna, C.; Rozic, R.; Nguyen, B.T.; Parsons, B.; Midura, R.J.; Lee, Y.-S. Differential Adaptations of the Musculoskeletal System after Spinal Cord Contusion and Transection in Rats. J. Neurotrauma 2018, 35, 1737–1744.
- Marini, S.; Barone, G.; Masini, A.; Dallolio, L.; Bragonzoni, L.; Longobucco, Y.; Maffei, F. The Effect of Physical Activity on Bone Biomarkers in People with Osteoporosis: A Systematic Review. Front. Endocrinol. 2020, 11, 585689.
- Craven, B.C.; Cirnigliaro, C.M.; Carbone, L.D.; Tsang, P.; Morse, L.R. The Pathophysiology, Identification and Management of Fracture Risk, Sublesional Osteoporosis and Fracture among Adults with Spinal Cord Injury. J. Pers. Med. 2023, 13, 966.
- Varacallo, M.; Davis, D.D.; Pizzutillo, P. Osteoporosis in Spinal Cord Injuries. In StatPearls; Ineligible Companies: Treasure Island, FL, USA, 2023.
- Edwards, W.B.; Schnitzer, T.J. Bone Imaging and Fracture Risk after Spinal Cord Injury. Curr. Osteoporos. Rep. 2015, 13, 310–317.
- Maïmoun, L.; Gelis, A.; Serrand, C.; Mura, T.; Humbert, L.; Boudousq, V.; de Santa-Barbara, P.; Laux, D.; Fattal, C.; Mariano-Goulart, D. Alteration of Volumetric Bone Mineral Density Parameters in Men with Spinal Cord Injury. Calcif. Tissue Int. 2023, 113, 304–316.
- Cirnigliaro, C.M.; La Fountaine, M.F.; Parrott, J.S.; Kirshblum, S.C.; Sauer, S.J.; Shapses, S.A.; McClure, I.A.; Bauman, W.A. Loss of lower extremity bone mineral density 1 year after denosumab is discontinued in persons with subacute spinal cord injury. Osteoporos. Int. 2023, 34, 741–748.
- Zhang, L.; Yin, Y.; Guo, J.; Jin, L.; Hou, Z. Chronic intermittent hypobaric hypoxia ameliorates osteoporosis after spinal cord injury through balancing osteoblast and osteoclast activities in rats. Front. Endocrinol. 2023, 14, 1035186.
- Ishimoto, R.; Mutsuzaki, H.; Shimizu, Y.; Kishimoto, H.; Takeuchi, R.; Hada, Y. Prevalence of Sarcopenic Obesity and Factors Influencing Body Composition in Persons with Spinal Cord Injury in Japan. Nutrients 2023, 15, 473.
- Bitra, A.; Doukov, T.; Croft, M.; Zajonc, D.M. Crystal structures of the human 4-1BB receptor bound to its ligand 4-1BBL reveal covalent receptor dimerization as a potential signaling amplifier. J. Biol. Chem. 2018, 293, 9958–9969.
- Wan, D.; Ai, S.; Ouyang, H.; Cheng, L. Activation of 4-1BB signaling in bone marrow stromal cells triggers bone loss via the p-38 MAPK-DKK1 axis in aged mice. Exp. Mol. Med. 2021, 53, 654–666.
- Cheng, P.; Liao, H.-Y.; Zhang, H.-H. The role of Wnt/mTOR signaling in spinal cord injury. J. Clin. Orthop. Trauma 2022, 25, 101760.
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3.
- Gifre, L.; Vidal, J.; Carrasco, J.L.; Filella, X.; Ruiz-Gaspà, S.; Muxi, A.; Portell, E.; Monegal, A.; Guañabens, N.; Peris, P. Effect of Recent Spinal Cord Injury on Wnt Signaling Antagonists (Sclerostin and Dkk-1) and Their Relationship with Bone Loss. A 12-Month Prospective Study. J. Bone Miner. Res. 2015, 30, 1014–1021.
- Sutor, T.W.; Kura, J.; Mattingly, A.J.; Otzel, D.M.; Yarrow, J.F. The Effects of Exercise and Activity-Based Physical Therapy on Bone after Spinal Cord Injury. Int. J. Mol. Sci. 2022, 23, 608.
- Yarrow, J.F.; Wnek, R.D.; Conover, C.F.; Reynolds, M.C.; Buckley, K.H.; Kura, J.R.; Sutor, T.W.; Otzel, D.M.; Mattingly, A.J.; Croft, S.; et al. Bone loss after severe spinal cord injury coincides with reduced bone formation and precedes bone blood flow deficits. J. Appl. Physiol. 2021, 131, 1288–1299.
- Morse, L.; Teng, Y.D.; Pham, L.; Newton, K.; Yu, D.; Liao, W.-L.; Kohler, T.; Müller, R.; Graves, D.; Stashenko, P.; et al. Spinal cord injury causes rapid osteoclastic resorption and growth plate abnormalities in growing rats (SCI-induced bone loss in growing rats). Osteoporos. Int. 2008, 19, 645–652.
- Le, B.; Ray, C.; Gonzalez, B.; Miskevics, S.; Weaver, F.M.; Priebe, M.; Carbone, L.D. Laboratory evaluation of secondary causes of bone loss in Veterans with spinal cord injury and disorders. Osteoporos. Int. 2019, 30, 2241–2248.
- Ma, Z.; Ma, M.; He, Y.; Sun, H.; Yang, B.; Dong, H.; Wang, Y. Bisphosphonates Alleviate Bone Loss in People with Acute Spinal Cord Injury: A Systematic Review and Meta-Analysis. World Neurosurg. 2023, 170, e584–e595.
- Ducher, G.; Courteix, D.; Même, S.; Magni, C.; Viala, J.; Benhamou, C. Bone geometry in response to long-term tennis playing and its relationship with muscle volume: A quantitative magnetic resonance imaging study in tennis players. Bone 2005, 37, 457–466.
- Zhu, S.; Chen, M.; Ying, Y.; Wu, Q.; Huang, Z.; Ni, W.; Wang, X.; Xu, H.; Bennett, S.; Xiao, J.; et al. Versatile subtypes of pericytes and their roles in spinal cord injury repair, bone development and repair. Bone Res. 2022, 10, 30.
- Bauman, W.A.; Emmons, R.R.; Cirnigliaro, C.M.; Kirshblum, S.C.; Spungen, A.M. An effective oral vitamin D replacement therapy in persons with spinal cord injury. J. Spinal Cord Med. 2011, 34, 455–460.
- Xu, H.; Chen, F.; Liu, T.; Xu, J.; Li, J.; Jiang, L.; Wang, X.; Sheng, J. Ellagic acid blocks RANKL–RANK interaction and suppresses RANKL-induced osteoclastogenesis by inhibiting RANK signaling pathways. Chem. Biol. Interact. 2020, 331, 109235.
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 6910312.
- Liu, F.-L.; Chen, C.-L.; Lee, C.-C.; Wu, C.-C.; Hsu, T.-H.; Tsai, C.-Y.; Huang, H.-S.; Chang, D.-M. The Simultaneous Inhibitory Effect of Niclosamide on RANKL-Induced Osteoclast Formation and Osteoblast Differentiation. Int. J. Med. Sci. 2017, 14, 840–852.
- Ashe, M.; Craven, C.; Eng, J.; Krassioukov, A. Prevention and Treatment of Bone Loss After a Spinal Cord Injury: A Systematic Review. Top. Spinal Cord Inj. Rehabil. 2007, 13, 123–145.
- McDonald, C.L.; Lemme, N.J.; Testa, E.J.; Aaron, R.; Hartnett, D.A.; Cohen, E.M. Bisphosphonates in Total Joint Arthroplasty: A Review of Their Use and Complications. Arthroplast. Today 2022, 14, 133–139.
- Nardone, V.; D'Asta, F.; Brandi, M.L. Pharmacological management of osteogenesis. Clinics 2014, 69, 438–446.
- Bauman, W.A. Pharmacological approaches for bone health in persons with spinal cord injury. Curr. Opin. Pharmacol. 2021, 60, 346–359.
- Russell, R.G.G. Bisphosphonates: Mode of Action and Pharmacology. Pediatrics 2007, 119 (Suppl. 2), S150–S162.
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045.
- Liu, X.; Liu, M.; Turner, R.; Iwaniec, U.; Kim, H.; Halloran, B. Dried plum mitigates spinal cord injury-induced bone loss in mice. JOR Spine 2020, 3, e1113.
- Edwards, W.B.; Simonian, N.; Haider, I.T.; Anschel, A.S.; Chen, D.; Gordon, K.E.; Gregory, E.K.; Kim, K.H.; Parachuri, R.; Troy, K.L.; et al. Effects of Teriparatide and Vibration on Bone Mass and Bone Strength in People with Bone Loss and Spinal Cord Injury: A Randomized, Controlled Trial. J. Bone Miner. Res. 2018, 33, 1729–1740.
- Kostenuik, P.J.; Nguyen, H.Q.; McCabe, J.; Warmington, K.S.; Kurahara, C.; Sun, N.; Chen, C.; Li, L.; Cattley, R.C.; Van, G.; et al. Denosumab, a Fully Human Monoclonal Antibody to RANKL, Inhibits Bone Resorption and Increases BMD in Knock-In Mice That Express Chimeric (Murine/Human) RANKL*. J. Bone Miner. Res. 2009, 24, 182–195.
- Guo, Y.; Guo, T.; Di, Y.; Xu, W.; Hu, Z.; Xiao, Y.; Yu, H.; Hou, J. Pharmacokinetics, pharmacodynamics, safety and immunogenicity of recombinant, fully human anti-RANKL monoclonal antibody (MW031) versus denosumab in Chinese healthy subjects: A single-center, randomized, double-blind, single-dose, parallel-controlled trial. Expert Opin. Biol. Ther. 2023, 23, 705–715.
- Won, K.Y.; Kalil, R.K.; Kim, Y.W.; Park, Y.-K. RANK signalling in bone lesions with osteoclast-like giant cells. Pathology 2011, 43, 318–321.
- Miyagawa, K.; Ohata, Y.; Delgado-Calle, J.; Teramachi, J.; Zhou, H.; Dempster, D.D.; Subler, M.A.; Windle, J.J.; Chirgwin, J.M.; Roodman, G.D.; et al. Osteoclast-derived IGF1 is required for pagetic lesion formation in vivo. JCI Insight 2020, 5.
- Song, R.; Gu, J.; Liu, X.; Zhu, J.; Wang, Q.; Gao, Q.; Zhang, J.; Cheng, L.; Tong, X.; Qi, X.; et al. Inhibition of osteoclast bone resorption activity through osteoprotegerin-induced damage of the sealing zone. Int. J. Mol. Med. 2014, 34, 856–862.
- Liau, L.L.; Looi, Q.H.; Chia, W.C.; Subramaniam, T.; Ng, M.H.; Law, J.X. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020, 10, 112.
- Abo-Aziza, F.A.; Zaki, A.K.A.; El-Maaty, A.M.A. Bone marrow-derived mesenchymal stem cell (BM-MSC): A tool of cell therapy in hydatid experimentally infected rats. Cell Regen. 2019, 8, 58–71.
- Boido, M.; Ghibaudi, M.; Gentile, P.; Favaro, E.; Fusaro, R.; Tonda-Turo, C. Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment. Sci. Rep. 2019, 9, 6402.
- Ning, Z.; Gu, P.; Zhang, J.; Cheung, C.W.; Lao, L.; Chen, H.; Zhang, Z.-J. Adiponectin regulates electroacupuncture-produced analgesic effects in association with a crosstalk between the peripheral circulation and the spinal cord. Brain Behav. Immun. 2021, 99, 43–52.
- Hook, M.A.; Falck, A.; Dundumulla, R.; Terminel, M.; Cunningham, R.; Sefiani, A.; Callaway, K.; Gaddy, D.; Geoffroy, C.G. Osteopenia in a Mouse Model of Spinal Cord Injury: Effects of Age, Sex and Motor Function. Biology 2022, 11, 189.
- Bergmann, P.; Body, J.J.; Boonen, S.; Boutsen, Y.; Devogelaer, J.P.; Goemaere, S.; Kaufman, J.; Reginster, J.Y.; Rozenberg, S. Loading and Skeletal Development and Maintenance. J. Osteoporos. 2010, 2011, 786752.
- Seo, B.R.; Mooney, D.J. Recent and Future Strategies of Mechanotherapy for Tissue Regenerative Rehabilitation. ACS Biomater. Sci. Eng. 2022, 8, 4639–4642.
- Takemura, Y.; Moriyama, Y.; Ayukawa, Y.; Kurata, K.; Rakhmatia, Y.D.; Koyano, K. Mechanical loading induced osteocyte apoptosis and connexin 43 expression in three-dimensional cell culture and dental implant model. J. Biomed. Mater. Res. Part A 2018, 107, 815–827.
- Harkema, S.J.; Ferreira, C.K.; Brand, R.J.v.D.; Krassioukov, A.V.; Jeffries, E.C.; Hoffman, S.M.; de Leon, R.; Dominguez, J.F.; Semerjian, T.Z.; Melgar, I.A.; et al. Improvements in Orthostatic Instability with Stand Locomotor Training in Individuals with Spinal Cord Injury. J. Neurotrauma 2008, 25, 1467–1475.
- Braaksma, J.M.; Vegter, R.J.; Leving, M.T.; van der Scheer, J.W.; Tepper, M.; Woldring, F.A.; van der Woude, L.H.; Houdijk, H.; de Groot, S. Handrim wheelchair propulsion technique in individuals with spinal cord injury with and without shoulder pain—A cross-sectional comparison. Am. J. Phys. Med. Rehabil. 2023, 102, 886–895.
- Lambach, R.L.; Stafford, N.E.; Kolesar, J.A.; Kiratli, B.J.; Creasey, G.H.; Gibbons, R.S.; Andrews, B.J.; Beaupre, G.S. Bone changes in the lower limbs from participation in an FES rowing exercise program implemented within two years after traumatic spinal cord injury. J. Spinal Cord Med. 2018, 43, 306–314.
- Bickel, C.S.; Yarar-Fisher, C.; Mahoney, E.T.; McCully, K.K. Neuromuscular Electrical Stimulation–Induced Resistance Training After SCI: A Review of the Dudley Protocol. Top. Spinal Cord Inj. Rehabil. 2015, 21, 294–302.
This entry is offline, you can click here to edit this entry!
