Leishmaniasis, a neglected tropical disease, encompasses a spectrum of clinical conditions and poses a significant risk of infection to over one billion people worldwide.
- cutaneous leishmaniasis
- Leishmania donovani
- skin lesions
- microscopy
- diagnosis
1. Introduction
2. The Diagnostic Tests Recommended by World Health Organization for Cutaneous Leishmaniasis
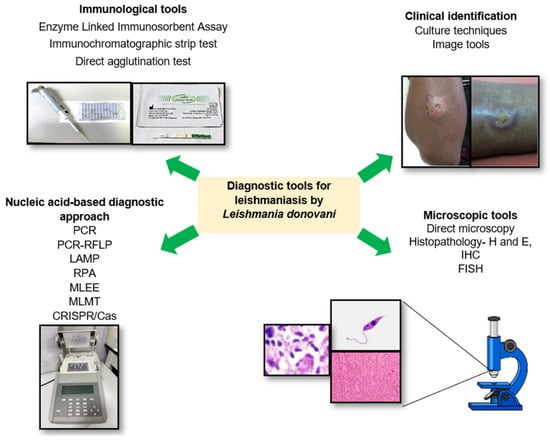
3. Clinical Identification
4. Direct Parasitological Methods for Diagnosis of CL
4.1. Direct Microscopy
4.2. Histopathology
4.3. Identification of Promastigotes by In Vitro Isolation (Parasite Cultures)
4.4. Supplementary Tests to Histopathology
5. Molecular Techniques for Identifying Leishmania Parasites
5.1. Polymerase Chain Reaction (PCR)
5.2. PCR–RFLP
5.3. LAMP
5.4. Recombinase Polymerase Amplification Assay (RPA)
5.5. Multi-Locus Enzyme Electrophoresis (MLEE)
5.6. Multi-Locus Microsatellite Typing (MLMT)
6. Immunological Techniques
6.1. Enzyme-Linked Immunosorbent Assay (ELISA) Based Diagnostics
6.2. Immunochromatographic Strip Test (ICT)
6.3. Direct Agglutination Test (DAT)
7. Challenges in Diagnosing Atypical Cutaneous Leishmaniasis Caused by Leishmania donovani
8. Innovative Approaches for Diagnosis of Cutaneous Leishmaniasis
Saavendra et al.’s 2020 [63] study in Peru revealed that high-frequency ultrasound offered a promising approach for the non-invasive visualization of Leishmania (Viannia) braziliensis-induced CL. Their findings demonstrated a strong correlation between ultrasound findings and histopathological CL characteristics, suggesting that high-frequency ultrasound could be a reliable diagnostic tool for CL in resource-limited settings.
Furthermore, an artificial intelligence (AI)-based algorithm is currently utilized for the automated detection and diagnosis of leishmaniasis. In 2022, Zare et al. [64] devised an algorithm for Leishmania parasite detection using integral image representation, facilitating faster processing. The study achieved a recall rate of 65% and a precision rate of 50% for detecting leishmania-infected macrophages. This tool’s versatility extends to identifying unusual patterns of atypical CL skin lesions.
Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein (CRISPR/Cas) systems are advanced tools for nucleic acid detection, offering high specificity, sensitivity, and speed. This technology is considered an ideal point-of-care test and it is versatile for various applications in the detection of CL in some geographical regions.
Buffi et al.’s 2023 study [65] presents a groundbreaking approach to improving leishmaniasis diagnostics with profound implications. Their utilization of high-resolution melting (HRM) analysis to pinpoint informative polymorphisms in single-copy genes encoding metabolic enzymes represents a significant leap forward, offering highly accurate and species-specific insights into Leishmania parasites, especially L. infantum. This precision is crucial for tailoring treatment strategies. Furthermore, the development of rapid genotyping assays based on HRM simplifies the genotyping process, replacing labor-intensive and specialized methods like multi-locus enzyme electrophoresis (MLEE) and multi-locus microsatellite typing (MLMT).
9. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13182989
References
- World Health Organization Factsheets. Leishmaniasis. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 16 June 2023).
- Patel, T.A.; Scadding, G.K.; Phillips, D.E.; Lockwood, D.N. Case report: Old world mucosal leishmaniasis: Report of five imported cases to the hospital for tropical diseases, London, United Kingdom. Am. J. Trop. Med. Hyg. 2017, 97, 1116.
- Thakur, L.; Singh, K.K.; Shanker, V.; Negi, A.; Jain, A.; Matlashewski, G.; Jain, M. Atypical leishmaniasis: A global perspective with emphasis on the Indian subcontinent. PLoS Neglected Trop. Dis. 2018, 12, e0006659.
- World Health Organization Global Health Observatory, Leishmaniasis. 2017. Available online: http://www.who.int/gho/neglected_diseases/leishmaniasis/en/ (accessed on 12 June 2023).
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M.D.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671.
- Gelanew, T.; Hurissa, Z.; Diro, E.; Kassahun, A.; Kuhls, K.; Schönian, G.; Hailu, A. Case report: Disseminated cutaneous leishmaniasis resembling post-kala-azar dermal leishmaniasis caused by Leishmania donovani in three patients co-infected with visceral leishmaniasis and human immunodeficiency virus/acquired immunodeficiency syndrome in Ethiopia. Am. J. Trop. Med. Hyg. 2011, 84, 906.
- Lata, S.; Kumari, S.; Das, R.; Pasi, S.; Dhiman, R.C. Typical and atypical cutaneous leishmaniasis in Himachal Pradesh (India). Heliyon 2021, 7, e07282.
- Krayter, L.; Bumb, R.A.; Azmi, K.; Wuttke, J.; Malik, M.D.; Schnur, L.F.; Salotra, P.; Schönian, G. Multilocus microsatellite typing reveals a genetic relationship but, also, genetic differences between Indian strains of Leishmania tropica causing cutaneous leishmaniasis and those causing visceral leishmaniasis. Parasites Vectors 2014, 7, 123.
- Kumar, N.P.; Srinivasan, R.; Anish, T.S.; Nandakumar, G.; Jambulingam, P. Cutaneous leishmaniasis caused by Leishmania donovani in the tribal population of the Agasthyamala Biosphere Reserve forest, Western Ghats, Kerala, India. J. Med. Microbiol. 2015, 64, 157–163.
- Sharma, N.L.; Mahajan, V.K.; Kanga, A.; Sood, A.; Katoch, V.M.; Mauricio, I.; Singh, C.D.; Parwan, U.C.; Sharma, V.K.; Sharma, R.C. Localized cutaneous leishmaniasis due to Leishmania donovani and Leishmania tropica: Preliminary findings of the study of 161 new cases from a new endemic focus in himachal pradesh, India. Am. J. Trop. Med. Hyg. 2005, 72, 819–824.
- Mebrahtu, Y.; Lawyer, P.; Githure, J.; Were, J.B.; Muigai, R.; Hendricks, L.; Leeuwenburg, J.; Koech, D.; Roberts, C. Visceral leishmaniasis unresponsive to pentostam caused by Leishmania tropica in Kenya. Am. J. Trop. Med. Hyg. 1989, 41, 289–294.
- Mebrahtu, Y.B.; Van Eys, G.; Guizani, I.; Lawyer, P.G.; Pamba, H.; Koech, D.; Roberts, C.; Perkins, P.V.; Were, J.B.; Hendricks, L.D. Human cutaneous leishmaniasis caused by Leishmania donovani sl in Kenya. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 598–601.
- Karunaweera, N.D.; Pratlong, F.; Siriwardane, H.V.Y.D.; Ihalamulla, R.L.; Dedet, J.P. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani zymodeme MON-37. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 380–381.
- Karunaweera, N.D. Leishmania donovani causing cutaneous leishmaniasis in Sri Lanka: A wolf in sheep’s clothing? Trends Parasitol. 2009, 25, 458–463.
- Ranasinghe, S.; Zhang, W.W.; Wickremasinghe, R.; Abeygunasekera, P.; Chandrasekharan, V.; Athauda, S.; Mendis, S.; Hulangamuwa, S.; Matlashewski, G.; Pratlong, F. Leishmania donovani zymodeme MON-37 isolated from an autochthonous visceral leishmaniasis patient in Sri Lanka. Pathog. Glob. Health 2012, 106, 421–424.
- Siriwardana, H.Y.; Noyes, H.A.; Beeching, N.J.; Chance, M.L.; Karunaweera, N.D.; Bates, P.A. Leishmania donovani and cutaneous leishmaniasis, Sri Lanka. Emerg. Infect. Dis. 2007, 13, 476.
- Elamin, E.M.; Guizani, I.; Guerbouj, S.; Gramiccia, M.; El Hassan, A.M.; Di Muccio, T.; Taha, M.A.; Mukhtar, M.M. Identification of Leishmania donovani as a cause of cutaneous leishmaniasis in Sudan. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 54–57.
- World Health Organization Factsheets. Diagnosis, Detection and Surveillance of Leishmaniasis. 2023. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/leishmaniasis/diagnosis (accessed on 27 August 2023).
- De Vries, H.J.; Schallig, H.D. Cutaneous leishmaniasis: A 2022 updated narrative review into diagnosis and management developments. Am. J. Clin. Dermatol. 2022, 23, 823–840.
- Ranawaka, R.R.; Abeygunasekara, P.H.; Weerakoon, H.S. Correlation of clinical, parasitological and histopathological diagnosis of cutaneous leishmaniasis in an endemic region in Sri Lanka. Ceylon Med. J. 2013, 57, 149.
- Siriwardana, H.V.Y.D.; Thalagala, N.; Karunaweera, N.D. Clinical and epidemiological studies on the cutaneous leishmaniasis caused by Leishmania (Leishmania) donovani in Sri Lanka. Ann. Trop. Med. Parasitol. 2010, 104, 213–223.
- Samaranayake, T.N.; Dissanayake, V.H.; Fernando, S.D. Clinical manifestations of cutaneous leishmaniasis in Sri Lanka—Possible evidence for genetic susceptibility among the Sinhalese. Ann. Trop. Med. Parasitol. 2008, 102, 383–390.
- Rajapaksa, U.S.; Ihalamulla, R.L.; Udagedera, C.; Karunaweera, N.D. Cutaneous leishmaniasis in southern Sri Lanka. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 799–803.
- Nawaratna, S.S.; Weilgama, D.J.; Wijekoon, C.J.; Dissanayake, M.; Rajapaksha, K. Cutaneous leishmaniasis, Sri Lanka. Emerg. Infect. Dis. 2007, 13, 1068.
- Siriwardana, H.V.Y.D.; Udagedara, C.U.; Karunaweera, N.D. Clinical features, risk factors and efficacy of cryotherapy in cutaneous leishmaniasis in Sri Lanka. Ceylon Med. J. 2003, 48, 10–12.
- Yadav, P.; Azam, M.; Ramesh, V.; Singh, R. Unusual Observations in Leishmaniasis—An Overview. Pathogens 2023, 12, 297.
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750.
- Remadi, L.; Haouas, N.; Chaara, D.; Slama, D.; Chargui, N.; Dabghi, R.; Jbeniani, H.; Mezhoud, H.; Babba, H. Clinical presentation of cutaneous leishmaniasis caused by Leishmania major. Dermatology 2017, 232, 752–759.
- Van Thiel, P.P.; Leenstra, T.; de Vries, H.J.; van der Sluis, A.; van Gool, T.; Krull, A.C.; van Vugt, M.; de Vries, P.J.; Zeegelaar, J.E.; Bart, A.; et al. Cutaneous leishmaniasis (Leishmania major infection) in Dutch troops deployed in northern Afghanistan: Epidemiology, clinical aspects, and treatment. Am. J. Trop. Med. Hyg. 2010, 83, 1295.
- Alraey, Y. Distribution and epidemiological features of cutaneous leishmaniasis in Asir Province, Saudi Arabia, from 2011 to 2020. J. Infect. Public Health 2022, 15, 757–765.
- Bousslimi, N.; Aoun, K.; Ben-Abda, I.; Ben-Alaya-Bouafif, N.; Raouane, M.; Bouratbine, A. Epidemiologic and clinical features of cutaneous leishmaniasis in southeastern Tunisia. Am. J. Trop. Med. Hyg. 2010, 83, 1034.
- Suprien, C.; Rocha, P.N.; Teixeira, M.; Carvalho, L.P.; Guimarães, L.H.; Bonvoisin, T.; Machado, P.R.; Carvalho, E.M. Clinical presentation and response to therapy in children with cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2020, 102, 777.
- Vieira-Gonçalves, R.; Pirmez, C.; Jorge, M.E.; Souza, W.J.; Oliveira, M.P.; Rutowitsch, M.S.; Da-Cruz, A.M. Clinical features of cutaneous and disseminated cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis in Paraty, Rio de Janeiro. Int. J. Dermatol. 2008, 47, 926–932.
- Guimaraes, L.H.; Queiroz, A.; Silva, J.A.; Silva, S.C.; Magalhaes, V.; Lago, E.L.; Machado, P.R.; Bacellar, O.; Wilson, M.E.; Beverley, S.M.; et al. Atypical manifestations of cutaneous leishmaniasis in a region endemic for Leishmania braziliensis: Clinical, immunological and parasitological aspects. PLoS Neglected Trop. Dis. 2016, 10, e0005100.
- Silveira, F.T.; Lainson, R.; De Castro Gomes, C.M.; Laurenti, M.D.; Corbett, C.E. Immunopathogenic competences of Leishmania (V.) braziliensis and L.(L.) amazonensis in American cutaneous leishmaniasis. Parasite Immunol. 2009, 31, 423–431.
- Andrade-Narvaez, F.J.; Medina-Peralta, S.; Vargas-Gonzalez, A.; Canto-Lara, S.B.; Estrada-Parra, S. The histopathology of cutaneous leishmaniasis due to Leishmania (Leishmania) mexicana in the Yucatan peninsula, Mexico. Rev. Inst. Med. Trop. Sao Paulo 2005, 47, 191–194.
- Andrade-Narváez, F.J.; Vargas-González, A.; Canto-Lara, S.B.; Damián-Centeno, A.G. Clinical picture of cutaneous leishmaniases due to Leishmania (Leishmania) mexicana in the Yucatan peninsula, Mexico. Mem. Inst. Oswaldo Cruz 2001, 96, 163–167.
- Siriwardana, H.V.Y.D.; Senarath, U.; Chandrawansa, P.H.; Karunaweera, N.D. Use of a clinical tool for screening and diagnosis of cutaneous leishmaniasis in Sri Lanka. Pathog. Glob. Health 2015, 109, 174–183.
- Kariyawasam, K.K.; Selvapandiyan, A.; Siriwardana, H.V.; Dube, A.; Karunanayake, P.; Senanayake, S.A.; Dey, R.; Gannavaram, S.; Nakhasi, H.L.; Karunaweera, N.D. Dermotropic Leishmania donovani in Sri Lanka: Visceralizing potential in clinical and preclinical studies. Parasitology 2018, 145, 443–452.
- Rajapaksa, U.S.; Ihalamulla, R.L.; Karunaweera, N.D. First report of mucosal tissue localisation of leishmaniasis in Sri Lanka. Ceylon Med. J. 2005, 50, 90–91.
- Siriwardana, H.V.Y.D.; Chandrawansa, P.H.; Sirimanna, G.; Karunaweera, N.D. Leishmaniasis in Sri Lanka: A decade old story. Sri Lankan J. Infect. Dis. 2012, 2, 2.
- Sundharkrishnan, L.; North, J.P. Histopathologic features of cutaneous leishmaniasis and use of CD1a staining for amastigotes in Old World and New World leishmaniasis. J. Cutan. Pathol. 2017, 44, 1005–1011.
- Riyal, H.; Samaranayake, N.; Amarathunga, P.; Munidasa, D.; Karunaweera, N.D. Histological findings associated with treatment response in cutaneous leishmaniasis: A clinicopathological correlation study. Int. J. Dermatol. 2023, 62, 1237–1247.
- Jayasena Kaluarachchi, T.; Wickremasinghe, R.; Weerasekera, M.; Yasawardene, S.; McBain, A.J.; Yapa, B.; De Silva, H.; Menike, C.; Jayathilake, S.; Munasinghe, A.; et al. Diagnosing human cutaneous leishmaniasis using fluorescence in situ hybridization. Pathog. Glob. Health 2021, 115, 307–314.
- Kocher, A.; Valiere, S.; Banuls, A.L.; Murienne, J. High-throughput sequencing of kDNA amplicons for the analysis of Leishmania minicircles and identification of Neotropical species. Parasitology 2018, 145, 585–594.
- Graça, G.C.; Volpini, A.C.; Romero, G.A.; Oliveira Neto, M.P.; Hueb, M.; Porrozzi, R.; Boité, M.C.; Cupolillo, E. Development and validation of PCR-based assays for diagnosis of American cutaneous leishmaniasis and identificatio nof the parasite species. Mem. Inst. Oswaldo Cruz 2012, 107, 664–674.
- De Silva, N.L.; De Silva, V.N.; Deerasinghe, A.T.; Rathnapala, U.L.; Itoh, M.; Takagi, H.; Weerasooriya, M.V.; Kato, H.; Yahathugoda, T.C. Development of a highly sensitive nested PCR and its application for the diagnosis of cutaneous leishmaniasis in Sri Lanka. Microorganisms 2022, 10, 990.
- Schönian, G.; Schnur, L.; El Fari, M.; Oskam, L.; Kolesnikov, A.A.; Sokolowska-Köhler, W.; Presber, W. Genetic heterogeneity in the species Leishmania tropica revealed by different PCR-based methods. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 217–224.
- Rai, T.; Shrestha, S.; Prajapati, S.; Bastola, A.; Parajuli, N.; Ghimire, P.G.; Bhandari, P.; Pandey, K.; Jain, M.; Matlashewski, G.; et al. Leishmania donovani persistence and transmission causing cutaneous leishmaniasis in unusual-foci of Nepal. Sci. Rep. 2023, 13, 12329.
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63.
- Kothalawala, H.S.; Karunaweera, N.D. Loop-mediated isothermal amplification assay as a sensitive diagnostic tool for Leishmania donovani infections in Sri Lanka. Ceylon Med. J. 2016, 61, 68.
- Gunaratna, G.; Manamperi, A.; Böhlken-Fascher, S.; Wickremasinge, R.; Gunawardena, K.; Yapa, B.; Pathirana, N.; Pathirana, H.; de Silva, N.; Sooriyaarachchi, M.; et al. Evaluation of rapid extraction and isothermal amplification techniques for the detection of Leishmania donovani DNA from skin lesions of suspected cases at the point of need in Sri Lanka. Parasites Vectors 2018, 11, 665.
- Ashton, F.E. Multilocus enzyme electrophoresis–application to the study of meningococcal meningitis and listeriosis. Can. J. Infect. Dis. Med. Microbiol. 1990, 1, 146–148.
- Alam, M.Z.; Haralambous, C.; Kuhls, K.; Gouzelou, E.; Sgouras, D.; Soteriadou, K.; Schnur, L.; Pratlong, F.; Schönian, G. The paraphyletic composition of Leishmania donovani zymodeme MON-37 revealed by multilocus microsatellite typing. Microbes Infect. 2009, 11, 707–715.
- Hartzell, J.D.; Aronson, N.E.; Weina, P.J.; Howard, R.S.; Yadava, A.; Wortmann, G.W. Positive rK39 serologic assay results in US servicemen with cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2008, 79, 843–846.
- Svobodová, M.; Alten, B.; Zídková, L.; Dvořák, V.; Hlavačková, J.; Myšková, J.; Šeblová, V.; Kasap, O.E.; Belen, A.; Votýpka, J.; et al. Cutaneous leishmaniasis caused by Leishmania infantum transmitted by Phlebotomus tobbi. Int. J. Parasitol. 2009, 39, 251–256.
- Molinet, F.J.; Ampuero, J.S.; Costa, R.D.; Noronha, E.F.; Romero, G.A. Specificity of the rapid rK39 antigen-based immunochromatographic test Kalazar Detect (r) in patients with cutaneous leishmaniasis in Brazil. Mem. Inst. Oswaldo Cruz 2013, 108, 293–296.
- Van Henten, S.; Fikre, H.; Melkamu, R.; Dessie, D.; Mekonnen, T.; Kassa, M.; Bogale, T.; Mohammed, R.; Cnops, L.; Vogt, F.; et al. Evaluation of the CL detect rapid test in Ethiopian patients suspected for cutaneous leishmaniasis. PLoS Neglected Trop. Dis. 2022, 16, e0010143.
- De Silva, G.; Somaratne, V.; Senaratne, S.; Vipuladasa, M.; Wickremasinghe, R.; Wickremasinghe, R.; Ranasinghe, S. Efficacy of a new rapid diagnostic test kit to diagnose Sri Lankan cutaneous leishmaniasis caused by Leishmania donovani. PLoS ONE 2017, 12, e0187024.
- Adams, E.R.; Jacquet, D.; Schoone, G.; Gidwani, K.; Boelaert, M.; Cunningham, J. Leishmaniasis direct agglutination test: Using pictorials as training materials to reduce inter-reader variability and improve accuracy. PLoS Neglected Trop. Dis. 2012, 6, e1946.
- Ahmed, Z.; Chowdhury, S.A.; Bhuiyan, S.I. Cutaneous leishmaniasis. Mymensingh Med. J. MMJ 2009, 18, 260–263.
- Siriwardana, H.V.Y.D.; Deepachandi, B.; Gunasekara, C.; Warnasooriya, W.; Karunaweera, N.D. Leishmania donovani induced cutaneous leishmaniasis: An insight into atypical clinical variants in Sri Lanka. J. Trop. Med. 2019, 2019, 4538597.
- Saavedra, A.C.; Valencia, B.M.; Tueros, P.; Wortsman, X.; Llanos-Cuentas, A.; Lavarello, R.J. Ultrasonographic characteristics of cutaneous leishmaniasis. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, e193.
- Zare, M.; Akbarialiabad, H.; Parsaei, H.; Asgari, Q.; Alinejad, A.; Bahreini, M.S.; Hosseini, S.H.; Ghofrani-Jahromi, M.; Shahriarirad, R.; Amirmoezzi, Y.; et al. A machine learning-based system for detecting leishmaniasis in microscopic images. BMC Infect. Dis. 2022, 22, 48.
- Buffi, G.; Ceccarelli, M.; Diotallevi, A.; Abruzzese, M.; Bruno, F.; Castelli, G.; Vitale, F.; Andreoni, F.; Bencardino, D.; Magnani, M.; et al. High-resolution melting (HRM)-based detection of polymorphisms in the malic enzyme and glucose-6-phosphate isomerase genes for Leishmania infantum genotyping. Parasites Vectors 2023, 16, 282.
