MSCC remains a challenging oncological emergency and requires effective multidisciplinary management for optimal effects on patients’ morbidity and quality of life. Diagnosis and prompt treatment can be difficult due to patient, clinician, and institutional factors. MSCC can present with a range of symptoms from minor sensory, motor, or autonomic disturbances to severe pain and complete paraplegia.
MSCC is a complication of cancer that occurs in 5–10% of patients and can particularly complicate the final stages of their disease. However, it could also be the presenting symptom of a malignancy; in a retrospective cohort study, 21% of patients with MSCC had no diagnosis of cancer within the last year. The exact incidence of cases in England and Wales is not clarified, as cases are not systematically recorded, but the NICE guideline approximates cases to be 4000 in England and Wales annually. The median age of diagnosis is 65 years and 60% of cases are found in lung, breast, and prostate cancers.
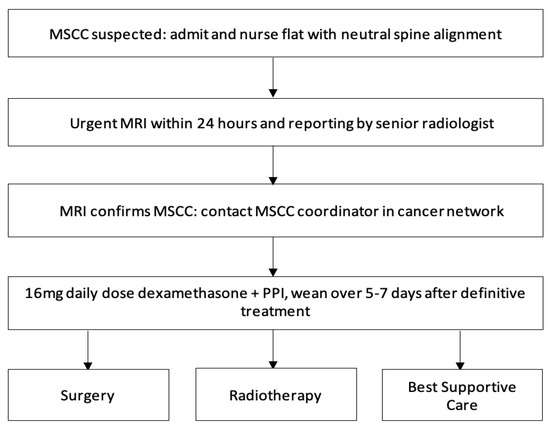
Studies have established that the mobility of patients at the time of diagnosis is a significant prognostic factor of mobility after MSCC treatment. Therefore, to avoid serious neurological implications of MSCC, it is crucial that diagnosis is made as early as possible. There are clear recommendations that patients should have rapid access to MRI, appropriate surgery, and RT, under an MSCC coordinator. Once the diagnosis of MSCC is suspected, patients with neurological deficits should receive prompt administration of dexamethasone. Local management strategies generally include palliative RT, or surgical posterior decompression with or without instrumentation or total en bloc spondylectomy. This audit study aimed to determine whether the current practice and management of MSCC at Medway, United Kingdom (UK), reflected the National Institute for Health and Care Excellence (NICE) guidelines.
Discussion
Diagnosing and treating MSCC as an oncological emergency remains critical to preserving neurological function, quality of life, and survival for our patients. Our results demonstrated that multiple features of our clinical practice fulfilled the guidelines. However, there was an overall delay in recognizing and establishing a diagnosis of MSCC and involving relevant members of the oncology team, all of which led to a delay in definitive treatment.
For earlier diagnosis, medical knowledge and understanding of the symptoms and signs of MSCC are essential. Back pain is commonly the first symptom of MSCC and occurs in up to 95% of patients; it can begin two to four months before progression of other neurological symptoms. The pain, either localised or radicular, usually increases in severity over time and can be worsened on coughing or lying down due to increased pressure and distension of the epidural plexus. The NICE guideline advises that MSCC should be considered in patients with cancer that have severe unremitting or nocturnal pain in the cervical, thoracic, or lumbar spine. Unfortunately, literature suggests a lack of awareness of the pain in early stages of MSCC in primary and secondary care. This is due to cancer patients having a different significance and type of pain compared to pain in non-cancer patients—for example, their pain could be attributed to tumour progression.
Our study highlights this challenge of early diagnosis. Only 40% of our 53 patients entering the MSCC pathway were suspected of having MSCC in the first 24 h. Late suspicion of MSCC was highlighted by 6% suspected after 72 h with the longest suspected after six days. These results highlight that our institution is potentially missing an opportunity for early diagnosis, increasing the serious risk of loss of neurological function. This is further demonstrated by 13% of patients never suspected of MSCC but diagnosed on imaging. Though patients may present from a variety of referral pathways, 83% of this patient group initially presented via A&E. The majority of suspicion of MSCC in 24 h would be by doctors in this department, along with the acute medical team afterwards. Based on this, optimising education for doctors in these specialties would be vital to improving our speed in detecting MSCC within 24 h. Furthermore, it seems that there is a delay in referring to the acute oncology service, as only 46% of referrals were completed within the first 24 h of admission. Many patients are referred once an MRI confirms MSCC. However, 98% of patients are promptly reviewed by the acute oncology service within 24 h or on the next working day. The NICE guideline emphasizes referral to an MSCC coordinator within 24 h and we must improve our practice significantly. Increased education of the management of MSCC and the role of acute oncology service in the A&E and acute medical teams may encourage earlier referral and a lower threshold for investigations leading to earlier diagnosis and treatment. Increased education for doctors on these issues is also well recommended in the literature.
Other symptoms of MSCC can be divided into motor, sensory, and autonomic deficits. Limb weakness can be the second most common symptom of MSCC as it affects 60–85% of patients. Patients report a progressive change in their gait or weakness over days or weeks, which can be difficult to appreciate if regular clinical reviews are not undertaken. Furthermore, White et al. found that 50% of patients only presented when their mobility was affected, despite experiencing other symptoms for over two months. This neurological change is considered an emergency for patients and doctors compared to other preceding symptoms, which further implicates diagnostic and treatment delay. Similarly, symptoms which can indicate a late consequence of MSCC are perineal anaesthesia in a saddle distribution and bladder and bowel dysfunction—this could be urinary retention, urinary or faecal incontinence, and constipation. Sensory symptoms are less common but patients may report paraesthesia extending up to 5 dermatomes below the level of compression. A limitation of our data is less focus on symptoms experienced by patients and examinations performed by doctors to exclude MSCC. This includes neurological examination and patients’ ability to walk—which is often missed out in initial assessment of MSCC. This would be vital to confirm in order to further understand the diagnostic delays found in our results. It would also provide context for educational training given to doctors and areas for improvement.
Contact the MSCC coordinator urgently (within 24 h) to discuss the care of patients with cancer and any of the following symptoms suggestive of spinal metastases:
- 1.
-
Pain in the middle (thoracic) or upper (cervical) spine
- 2.
-
Progressive lower (lumbar) spinal pain
- 3.
-
Severe unremitting lower spinal pain
- 4.
-
Spinal pain aggravated by straining (for example, at stool, or when coughing or sneezing)
- 5.
-
Localised spinal tenderness
- 6.
-
Nocturnal spinal pain preventing sleep
Contact the MSCC coordinator immediately to discuss the care of patients with cancer and symptoms suggestive of spinal metastases who have any of the following neurological symptoms or signs suggestive of MSCC, and view them as an oncological emergency:
- 1.
-
Neurological symptoms including radicular pain, any limb weakness, difficulty in walking, sensory loss or bladder or bowel dysfunction
- 2.
-
Neurological signs of spinal cord or cauda equina compression
Perform frequent clinical reviews of patients with cancer who develop lower spinal pain that is clinically thought to be of non-specific origin (that is, it is not progressive, severe or aggravated by straining and has no accompanying neurological symptoms). In particular, look for:
- [1]
-
Development of progressive pain or other symptoms suggestive of spinal metastases (contact the MSCC coordinator within 24 h), or
- [2]
-
Development of neurological symptoms or signs suggestive of MSCC (contact the MSCC coordinator immediately)
Perform frequent clinical reviews of patients without a prior diagnosis of cancer who develop suspicious spinal pain with or without neurological symptoms. Treat or refer patients with stable and mild symptoms by normal non-specific spinal pathways, or refer by cancer pathway if concerned. In particular, look for:
- 1.
-
Development of progressive pain or other symptoms suggestive of spinal metastases (contact the MSCC coordinator within 24 h), or
- 2.
-
Development of neurological symptoms or signs suggestive of MSCC (contact the MSCC coordinator immediately)
Timely access to MRI imaging will also improve diagnostic delays of MSCC. As per the NICE guideline, MRI remains the gold-standard to diagnosing MSCC with sensitivity of 93% and specificity of 97% and should be done within 24 h. Unfortunately, literature shows that despite the non-invasive and highly effective investigation choice of MRI, many patients are still being diagnosed late with this. This differs from our study, where 91% of patients suspected of MSCC had their MRI within 24 h. However, this is reduced with 64% of patients receiving MRI within 24 h of admission. Consequently, the rapid availability of MRI imaging in our centre complies with the guidelines; however, there is a gap of clinical suspicion of MSCC when patients are admitted. This again links back to the delay in diagnosis of MSCC reported in the literature, which could be due to the history taking and clinical examination performed by doctors. Our objective should be to improve MRI imaging in MSCC patients so that it is within 24 h of admission.
Another issue highlighted by our data was timing of reporting of MRI. There is no explicit time cut-off for this in the guidelines nor evidence in the literature; however, if both MRI and treatment need to be initiated within 24 h of suspicion, then delays in reporting will lead to delays in diagnosis. This can be further complicated if MRIs are reported out-of-hours and not picked up in time by the on-call team. Literature already demonstrates that there is a low percentage of patients diagnosed with MSCC along with its sub-optimal management on the weekends. One of our patients had an MRI as an outpatient with a report only completed 72 h after. Though RT was delivered on the day the MRI was reported, there was still a delay which could be significant for symptom progression and consequently success of treatment. These delays could be due to the limited number of radiologists who are able to report MRIs in the hospital but this needs to be explored further. Therefore, to allow for treatment of MSCC as soon as possible, oncology centres should perhaps outline a clear pathway of when MRI should be performed and also reported by a senior radiologist. Furthermore, this pathway should include an MRI-imaging grading system of MSCC.
As well as early diagnosis, treatment of MSCC should be delivered within 24 h. The treatment is a combination of high-dose steroids, RT, surgical intervention, and extensive rehabilitation, and must be initiated within 24 h of diagnosis to prevent further neurological decline . Our study shows significant improvement is needed with only 36% of patients receiving treatment less than 24 h after MRI diagnosis. While awaiting definitive management, such as RT and surgery, high doses of steroids provide analgesia, decrease spinal cord vasogenic oedema, and the secondary complication of reduced arterial flow and therefore, prevent further neurological deterioration. In some cases, it can decompress the tumour causing the compression. Steroids should be given immediately within 12 h of diagnosis for optimum efficacy and weaned after RT or surgery over 5–7 days to avoid side-effects. Our study could be improved by inclusion of when steroids were administered, inclusion of Proton Pump Inhibitors (PPIs), and whether they were adequately weaned following definitive treatment.
Surgery is indicated in patients for surgical decompression and spinal stabilisation. Surgical decompression followed by adjuvant RT has shown more favourable outcomes than RT alone in the literature. Patients should have a prognosis of more than six months to be considered for surgery. However, surgery can lead to complications such as pulmonary embolism, infections including postoperative pneumonia, cerebrospinal fluid leaks, and major bleeding. This may account for reluctance of neurosurgeons to operate and explain the findings in our study with 21% of patients receiving surgery at a tertiary care hospital.
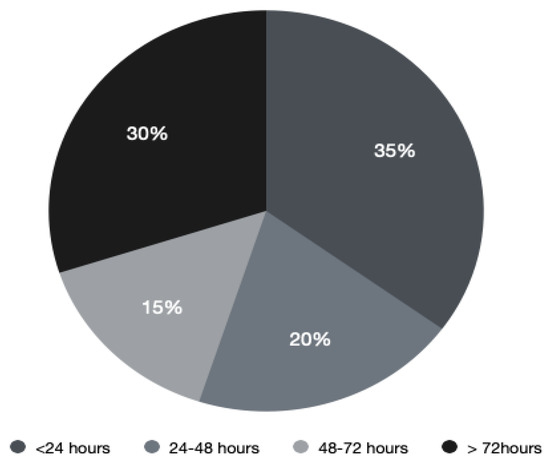
RT is highly effective in MSCC by providing analgesia and preventing further neurological deterioration. It is indicated within 24 h of diagnosis and can provide benefit to patients who are not surgical candidates. Fractions of RT given depend on the primary malignancy and its systemic burden, duration of symptoms, and prognosis. The majority of patients in our study (20 patients, 69%) received RT and this was also influenced by performance status and life expectancy of patients. However, only 35% were irradiated less than 24 h after MRI diagnosis.
Time from confirmed malignant spinal cord compression (MSCC) to radiotherapy (RT).
It is clear that a MSCC referral pathway needs to be more streamlined for improved treatment outcomes. Clinical oncologists should be included as part of the neurosurgical pathway recommended above in order to improve communication between three different areas of expertise and treatment timing. It may be advisable to have an MSCC coordinator in the oncology centre along with representatives from both the clinical oncologist and neurosurgical teams to oversee the treatment pathway and improve clinical practice. This could be further implemented in an MDM for MSCC mid-week compared to later on. Having an MSCC coordinator could improve both the diagnostic and treatment pathways. In our study, delays in RT were also caused by MSCC confirmed out-of-hours or over the weekend, which led to late referrals to the acute oncology service, clinical oncologist and neurosurgical teams. An MSCC coordinator could provide teaching to junior doctors on the referral process and treatment pathway as part of their core teaching curriculum. Furthermore, referral forms can be created, which include clinical history, assessment, MRI report, and contact details of the neurosurgical and clinical oncologist teams to encourage A&E and medical specialties to refer to them all simultaneously. This form can then be emailed to the MSCC coordinator who can act as the primary point of referral to these specialties. A defined pathway such as this will improve access to definitive treatment and consequently improve neurological outcomes.
Conclusions and Future Directions
MSCC represents an oncological emergency and clinicians should be aware of the potential long-term neurological impact. Urgent diagnosis and treatment is still challenging. MRI of the whole spine is the imaging method of choice that should be carried out within 24 h of clinical suspicion. Steroid therapy is administered immediately after the establishment of diagnosis, followed by definitive treatment, which may include any combination of surgery and/or RT. Treatment should ideally be initiated within 24 h of the confirmed MSCC. Our study demonstrates that MSCC is overall poorly understood amongst clinicians. It is evident that trainees require further teaching to improve their knowledge. Equally, oncological patients should be aware of the signs and symptoms of MSCC in order to optimise early detection.
In summary, formulation of a standard treatment protocol may be beneficial in assessing, auditing, and improving the standard of care in the acute management of patients presenting with MSCC. To aid this, we have developed an electronic MSCC form in the oncology Electronic Patient Record (EPR) system to document the management of MSCC more accurately. Furthermore, updated guidelines have been written to provide clearer guidance to the clinical teams seeing and assessing these patients when they first present in our hospital. To avoid diagnostic and therapeutic delays, early referral to the local acute oncology team to co-ordinate the patient pathway is critical. Overall, the gold standard pathway would include a dedicated team, including a coordinator, radiologist, clinical oncologist, and neurosurgeon to oversee the treatment pathway and improve clinical practice.