Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Nanotechnology has ushered in a new era of medical innovation, offering unique solutions to longstanding healthcare challenges. Among nanomaterials, copper and copper oxide nanoparticles stand out as promising candidates for a multitude of medical applications.
- copper nanoparticles
- drug delivery systems
- cancer therapy
1. Introduction
During the past decade, the development of nanomaterials has expanded into a wide range of clinical applications. To date, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved approximately 100 nanomedicines, while more than 500 are additionally in clinical trials (Phase I or II) [1][2]. Nanomaterial-based formulations are vital for the worldwide healthcare system [3][4][5]. Advanced nanomedicines can be used as drug vehicles to treat cancer, bacterial and viral infections, and neurological, cardiovascular, immunological, and respiratory system diseases [6][7][8][9][10]. Nanoformulations can be helpful in bioimaging and diagnosis techniques, for example as contrast agents in magnetic resonance imaging (MRI) or positron emission tomography (PET) tracers [11][12][13][14][15][16][17]. The main advantage of nanomedicines lies in their multimodality [18][19]. Nanomaterials simultaneously provide targeted detection and therapeutic capabilities, giving rise to a theranostic approach [20][21][22]. Additionally, nanomaterials can be used to tailor a therapeutic agent to a specific target in a individual patient with a particular disease, contributing to the development of personalised medicine [23][24].
As the field of nanomedicine continues to evolve, the incorporation of copper nanoparticles (NPs) offers exciting new possibilities for enhanced medical treatments and diagnostics. Copper is an essential trace element in all body tissues and is vital in various fundamental biochemical pathways such as glucose, cholesterol, and iron metabolism. It is required for cardiovascular integrity, elasticity of the lung, bone formation, and to work with iron to provide red blood cell synthesis [25]. Both a deficiency and an excess of copper can affect the body’s functions. An excess of copper can cause damage to the liver and various symptoms in the gastrointestinal tract, such as cramps, diarrhoea, vomiting, or abdominal pain. A deficiency can lead to problems with the cardiovascular system, anaemia, tissue damage, and bone defects [26]. Copper use is widespread globally due to its versatility and antimicrobial activity. Copper-based nanomaterials can be applied in agriculture, livestock, water treatment, wood preservation, and textile production. In addition, copper nanomaterials can serve as an alternative to the other noble metal nanomaterials in solar energy conversion, batteries, and electrochemical sensors due to their conductive capacity and lower production costs [27][28].
Copper and copper-based nanoparticles have excellent antibacterial activity against Staphylococcus aureus (including methicillin-resistant S. aureus), Bacillus subtilis, Proteus vulgaris, and Escherichia coli [29][30]. Copper-based nanoparticles were found to have even higher antibacterial properties than Ag NPs [31], and some reports have shown a synergistic antibacterial effect of copper or copper oxide(II) nanoparticles in combinations with silver nanoparticles [32][33].
The widespread use of copper-based nanoparticles in medical applications, including as antimicrobial or antiviral agents, has prompted researchers to expand their studies on the toxicity of copper nanomaterials to microorganisms, animals, and humans. Most scientific research indicates that the toxicity of copper-based nanoparticles is related to the accumulation of nanoparticles around the cell, their dissolution, and their subsequent adhesion to the cell membrane caused by electrostatic interaction. The adhesion of nanoparticles and subsequent release of copper ions disintegrate the cell membrane, facilitating the entry of copper nanoparticles and ions inside the cells. This release of copper ions can cause an increase in the reactive oxygen species (ROS) level, oxidation of proteins, reduced adenosine triphosphate (ATP) production, and DNA damage [34].
The diverse applications of copper nanoparticles increase the likelihood of their leakage into the environment. Once released, nanoparticles undergo various transformations, including dissolution, and can accumulate in plants and animals, causing cytotoxic effects. The toxicity of copper nanomaterials depends on the properties of the nanoparticles (such as size, shape, and surface properties) and the environmental conditions (i.e., composition, temperature, and pH of medium). Woźniak-Budych et al. investigated the stability of copper oxide(I) nanoparticles in various biological fluids, i.e., salivary fluids (pH 6.75), gastric fluid (pH 1.3), intestinal fluids (pH 6.0), and blood plasma (pH 7.2–7.4). They concluded that the stability of Cu2O NPs strongly depended on the fluid’s chemistry, i.e., pH, ionic strength, and presence of enzymes [35]. Nanoparticles formed agglomerates in all tested biological media; however, aggregation slowed the dissolution process. The highest dissolution and release of copper ions was detected in gastric and intestinal fluids.
2. Anticancer Potential of Copper Nanoformulations
Copper and copper oxide nanoparticles have drawn much interest in the biomedical fields, offering diverse benefits, including drug stability, proper biodistribution, improved therapeutic index, and active agent delivery to the specific site (active or passive targeting) [35][36][37][38][39]. For instance, copper nanoparticles can be utilised as anticancer therapeutics or effective drug nanocarriers due to their large specific surface area that facilitates conjugation with various biomolecules. Kang et al. [40] designed copper diethyldithiocarbamate nanoparticles (Cu(DDC)2 NPs) to overcome resistance in prostate cancer therapy. Based on in vivo studies (xenograft tumour model in male athymic nude mice), it was assumed that Cu(DDC)2 NPs prevent cancer cell metastasis and treat resistance by by-passing P-glycoprotein (P-gp) mediated drug efflux transporters, which are responsible for removal of anticancer drugs out of cells. Moreover, it was shown that Cu(DDC)2 NPs did not interfere with normal P-gp activities in the body, which is very important because these transporters play a vital role in toxin elimination in healthy tissues. Woźniak-Budych et al. [41][42] demonstrated that copper, copper oxide(I) nanoparticles, and copper–gold core–shell nanostructures exhibit anticancer potential against cervical cancer (HeLa cells, in vitro studies). They can act as therapeutic agents alone or as vehicles for doxorubicin, providing controlled anticancer drug release and inhibiting cancer cell proliferation [42]. The authors also investigated the stability aspect of copper oxide(I) nanoparticles in various physiological fluids, highlighting that glutathione- and hyaluronic acid functionalisation can reduce nanoparticle aggregate formation and corrosion in biological media [35].
The anticancer potential of copper oxide(II) nanoparticles (CuO NPs) against human breast cancer was the topic of studies by the Tabrez group [43]. Treatment with CuO NPs caused a significant decrease in breast cancer cell viability, morphological deformation of cancer cells, enhanced reactive oxygen species (ROS) production, and loss of mitochondrial membrane potential. It indicates that uptake of copper oxide(II) nanoparticles triggers apoptosis. However, copper and copper oxide nanoparticles can also suppress cancer cell viability by necrosis (see Figure 1) [44].
Chen and co-workers designed copper-based nanomaterials (Hc-CuO NPs) consisting of copper oxide(II) nanoparticles (40–45 nm in size) and herbal extract of Houttuynia cordata (Hc) [45]. The authors showed that Hc-CuO NPs inhibited the proliferation of cervical cancer in vitro by ROS overproduction and the induction of apoptosis by targeting PI3K/Akt (the phosphatidylinositol 3-kinase/protein kinase B) signalling pathways in cancer cells. The cytotoxicity of copper nanocomplexes against cervical cancer resulted from combining CuO NPs and plant extracts with strong antioxidant and anti-inflammatory activities. The synthesis of copper nanoparticles in the presence of natural bioactive compounds has also been investigated by Phull et al. [46] and Abdelhakm et al. [47]. Copper oxide(II) nanoparticles coated with fucoidan derived from Undaria Pinnatifida algae exhibited antiproliferative and genotoxic effects on HeLa cells. Additionally, fucoidan-modified CuO NPs demonstrated the ability to modulate the apoptosis of cancer cells via activation of apoptosis-related proteins, including B-cell lymphoma 2 (BCL2), Bcl-2-associated X protein (BAX), cleaved caspase-3, and cleaved poly(ADP-ribose) polymerase (PARP) [46]. In turn, copper nanoparticles functionalised with chrysin as a radiosensitiser improved the effect of radiation on Ehrlich ascites carcinoma in vivo [47]. The combination of Cu NPs and γ-radiation caused a reduction in tumour volume (Ehrlich tumour-bearing mice model) by stimulating apoptosis and inhibiting the nuclear factor-kappa B (NF-κB), p38 mitogen-activated protein kinase (p38 MAPK), and cyclin D1 gene expression. The anticancer potential of CuO NPs was the topic of Benguigui et al.’s research [48]. Copper oxide(II) nanoparticles have been shown to be effective in the treatment of human pancreatic cancer in vitro and in vivo (mice model). The inhibition of tumour growth was related to increased ROS levels and reduction of the mitochondrial potential of the cancer cell membrane. Scientists from China Pharmaceutical University published interesting in vivo research on encapsulated copper sulfide nanoparticles for combined photothermal and chemotherapy [49]. This drug delivery system consisted of copper sulfide nanoparticles, doxorubicin, and a near-infrared dye (NIR) dye (methylene blue analogue, MBA) encapsulated with stearic acid and lauric acid. In addition, to improve the stability of the encapsulated nanoformulation, their surface was covered with a hydrophilic shell made of lecithin and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine–polyethylene glycol 5000 (DSPE-PEG5000). The tumour growth (Ehrlich ascites carcinoma (EAC) tumour-bearing mice model) was significantly inhibited as a result of hyperthermia, triggered by the synergistic action of CuS and MBA (808 nm laser irradiation, 1.0 W/cm2) and the release of doxorubicin (DOX) upon thermal-induced decomposition of the outer shell. The tumour inhibition rate reached almost 68% after five days of injection of copper-based nanoformulation. These results indicate that copper sulfide nanoparticles are an effective photothermal agent in advanced combined anticancer therapy. The potential of copper nanoformulations as an anticancer agent has been extensively studied in recent years, with the continuous emergence of innovative approaches. As researchers explore the multifaceted applications of copper and copper oxide nanoparticles in oncology, the landscape of therapeutic possibilities continues to expand dynamically.
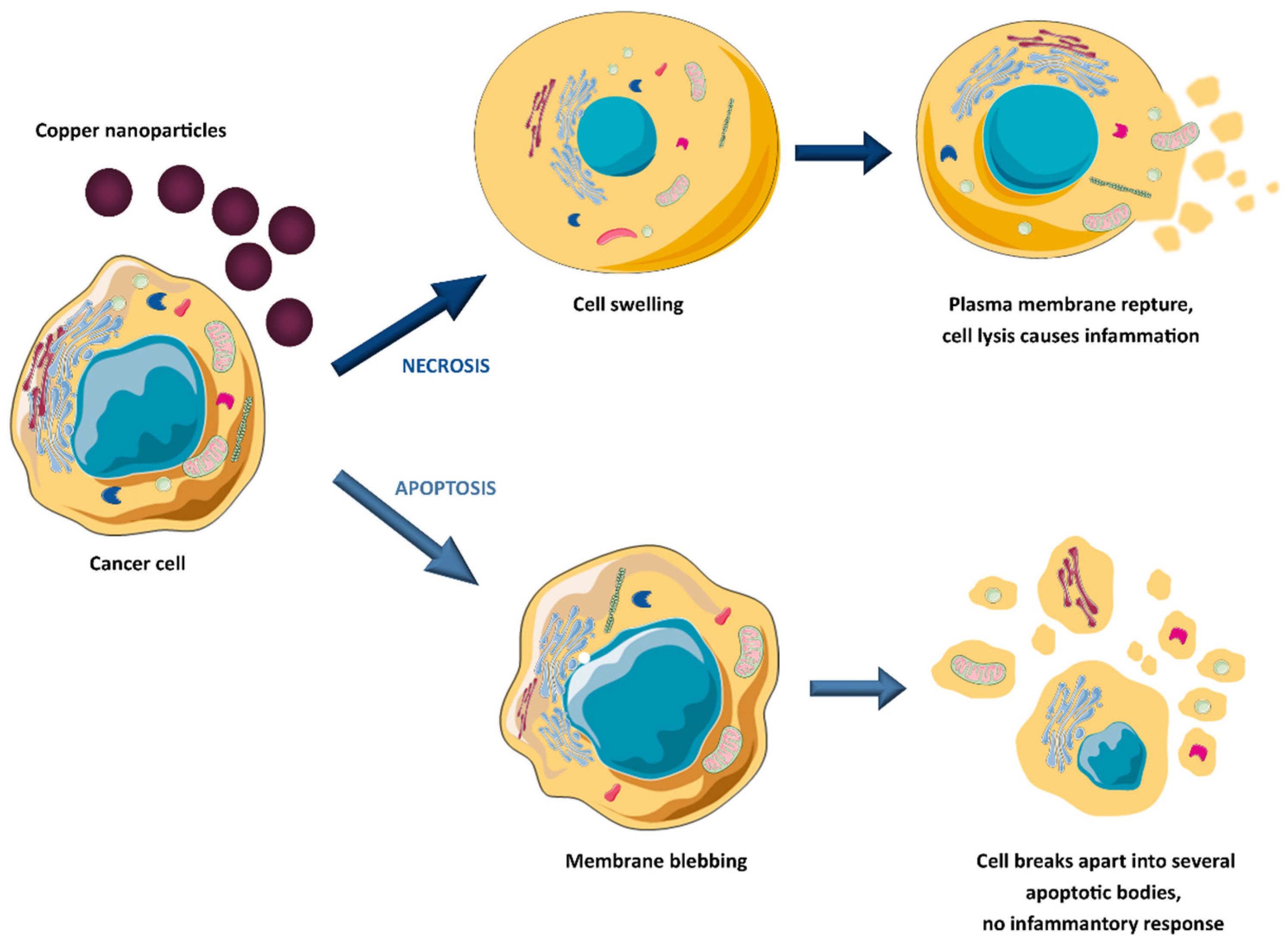
Figure 1. Copper-induced tumour cell death mechanisms.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28186687
References
- Thapa, R.K.; Kim, J.O. Nanomedicine-based commercial formulations: Current developments and future prospects. J. Pharm. Investig. 2022, 53, 19–33.
- Malviya, R.; Fuloria, S.; Verma, S.; Subramaniyan, V.; Sathasivam, K.V.; Kumarasamy, V.; Kumar, D.; Vellasamy, S.; Meenakshi, D.; Yadav, S.; et al. Commercial utilities and future perspective of nanomedicines. PeerJ 2021, 9, e12392.
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery (Review). Biomed. Rep. 2021, 14, 42.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Singh, C.K.; Sodhi, K.K. The emerging significance of nanomedicine-based approaches to fighting COVID-19 variants of concern: A perspective on the nanotechnology’s role in COVID-19 diagnosis and treatment. Front. Nanotechnol. 2023, 4, 1084033.
- Zhu, R.; Zhang, F.; Peng, Y.; Xie, T.; Wang, Y.; Lan, Y. Current Progress in Cancer Treatment Using Nanomaterials. Front. Oncol. 2022, 12, 930125.
- Sarkar, J.; Das, S.; Aich, S.; Bhattacharyya, P.; Acharya, K. Antiviral potential of nanoparticles for the treatment of Coronavirus infections. J. Trace Elem. Med. Biol. 2022, 72, 126977.
- Bayade, G.; Wu, M.R.; Massicotte, R.; Deryabin, D.G.E.; Yahia, L.H. Biocidal properties of copper nanoparticles. Eng. Biomat. 2021, 24, 2–17.
- Waris, A.; Ali, A.; Khan, A.U.; Asim, M.; Zamel, D.; Fatima, K.; Raziq, A.; Khan, M.A.; Akbar, N.; Baset, A.; et al. Applications of Various Types of Nanomaterials for the Treatment of Neurological Disorders. Nanomat 2022, 12, 2140.
- De Matteis, V.; Rizzello, L. Noble Metals and Soft Bio-Inspired Nanoparticles in Retinal Diseases Treatment: A Perspective. Cells 2020, 9, 679.
- Belderbos, S.; González-Gómez, M.A.; Cleeren, F.; Wouters, J.; Piñeiro, Y.; Deroose, C.M.; Coosemans, A.; Gsell, W.; Bormans, G.; Rivas, J.; et al. Simultaneous in vivo PET/MRI using fluorine-18 labeled Fe3O4@Al(OH)3 nanoparticles: Comparison of nanoparticle and nanoparticle-labeled stem cell distribution. EJNMMI Res. 2020, 10, 73.
- Thomas, G.; Boudon, J.; Maurizi, L.; Moreau, M.; Walker, P.; Severin, I.; Oudot, A.; Goze, C.; Poty, S.; Vrigneaud, J.-M.; et al. Innovative Magnetic Nanoparticles for PET/MRI Bimodal Imaging. ACS Omega 2019, 4, 2637–2648.
- Forte, E.; Fiorenza, D.; Torino, E.; Di Polidoro, A.C.; Cavaliere, C.; Netti, P.A.; Salvatore, M.; Aiello, M. Radiolabeled PET/MRI Nanoparticles for Tumor Imaging. J. Clin. Med. 2020, 9, 89.
- Miedema, I.H.C.; Zwezerijnen, G.J.C.; Huisman, M.C.; Doeleman, E.; Mathijssen, R.H.J.; Lammers, T.; Hu, Q.; van Dongen, G.A.M.S.; Rijcken, C.J.F.; Vugts, D.J.; et al. PET-CT Imaging of Polymeric Nanoparticle Tumor Accumulation in Patients. Adv. Mater. 2022, 34, e2201043.
- Wei, H.; Wiśniowska, A.; Fan, J.; Harvey, P.; Li, Y.; Wu, V.; Hansen, E.C.; Zhang, J.; Kaul, M.G.; Frey, A.M.; et al. Single-nanometer iron oxide nanoparticles as tissue-permeable MRI contrast agents. Proc. Natl. Acad. Sci. USA 2021, 118, e2102340118.
- Yang, H.; Wang, H.; Wen, C.; Bai, S.; Wei, P.; Xu, B.; Xu, Y.; Liang, C.; Zhang, Y.; Zhang, G.; et al. Effects of iron oxide nanoparticles as T2-MRI contrast agents on reproductive system in male mice. J. Nanobiotechnol. 2022, 20, 98.
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 49–91.
- Tsang, M.-K.; Wong, Y.-T.; Hao, J. Cutting-Edge Nanomaterials for Advanced Multimodal Bioimaging Applications. Small Methods 2018, 2, 1700265.
- Yáñez-Sedeño, P.; González-Cortés, A.; Campuzano, S.; Pingarrón, J.M. Multimodal/Multifunctional Nanomaterials in (Bio)electrochemistry: Now and in the Coming Decade. Nanomaterials 2020, 10, 2556.
- Zhu, W.; Wei, Z.; Han, C.; Weng, X. Nanomaterials as Promising Theranostic Tools in Nanomedicine and Their Applications in Clinical Disease Diagnosis and Treatment. Nanomaterials 2021, 11, 3346.
- Thangam, R.; Paulmurugan, R.; Kang, H. Functionalized Nanomaterials as Tailored Theranostic Agents in Brain Imaging. Nanomaterials 2021, 12, 18.
- Kashyap, B.K.; Singh, V.V.; Solanki, M.K.; Kumar, A.; Ruokolainen, J.; Kesari, K.K. Smart Nanomaterials in Cancer Theranostics: Challenges and Opportunities. ACS Omega 2023, 8, 14290–14320.
- Han, H.J.; Ekweremadu, C.; Patel, N. Advanced drug delivery system with nanomaterials for personalised medicine to treat breast cancer. J. Drug Deliv. Sci. Technol. 2019, 52, 1051–1060.
- Alghamdi, M.A.; Fallica, A.N.; Virzì, N.; Kesharwani, P.; Pittalà, V.; Greish, K. The Promise of Nanotechnology in Personalized Medicine. J. Pers. Med. 2022, 12, 673.
- Copper-Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/ (accessed on 15 September 2023).
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227.
- Bakshi, M.; Kumar, A. Applications of copper nanoparticles in plant protection and pollution sensing: Toward promoting sustainable agriculture. In Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–413.
- Harishchandra, B.D.; Pappuswamy, M.; Pu, A.; Shama, G.; Pragatheesh, A.; Arumugam, V.A.; Periyaswamy, T.; Sundaram, R. Copper Nanoparticles: A Review on Synthesis, Characterization and Applications. Asian Pac. J. Cancer Biol. 2020, 5, 201–210.
- Luong, H.T.; Nguyen, C.X.; Lam, T.T.; Nguyen, T.-H.; Dang, Q.-L.; Lee, J.-H.; Hur, H.-G.; Nguyen, H.T.; Ho, C.T. Antibacterial effect of copper nanoparticles produced in a Shewanella-supported non-external circuit bioelectrical system on bacterial plant pathogens. RSC Adv. 2022, 12, 4428–4436.
- Sadia, B.O.; Cherutoi, J.K.; Achisa, C.M. Optimization, Characterization, and Antibacterial Activity of Copper Nanoparticles Synthesized Using Senna didymobotrya Root Extract. J. Nanotechnol. 2021, 2021, 5611434.
- Doolotkeldieva, T.; Bobusheva, S.; Zhasnakunov, Z.; Satybaldiev, A. Biological Activity of Ag and Cu Monometallic Nanoparticles and Ag-Cu Bimetallic Nanocomposites against Plant Pathogens and Seeds. J. Nanomater. 2022, 2022, 1190280.
- Zhou, F.; Zhu, Y.; Yang, L.; Yang, D.-Q.; Sacher, E. Ag NP catalysis of Cu ions in the preparation of AgCu NPs and the mechanism of their enhanced antibacterial efficacy. Colloids Surf. A Physicochem. Eng. Asp. 2021, 632, 127831.
- Vasiliev, G.; Kubo, A.-L.; Vija, H.; Kahru, A.; Bondar, D.; Karpichev, Y.; Bondarenko, O. Synergistic antibacterial effect of copper and silver nanoparticles and their mechanism of action. Sci. Rep. 2023, 13, 9202.
- Ramos-Zúñiga, J.; Bruna, N.; Pérez-Donoso, J.M. Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. Int. J. Mol. Sci. 2023, 24, 10503.
- Woźniak-Budych, M.J.; Maciejewska, B.; Przysiecka, Ł.; Wieczorek, D.; Staszak, K.; Jenczyk, J.; Jesionowski, T.; Jurga, S. Comprehensive study of stability of copper oxide nanoparticles in complex biological media. J. Mol. Liq. 2020, 319, 114086.
- Singh, M.; Jagaran, K. Nanomedicine for COVID-19: Potential of Copper Nanoparticles. Biointerface Res. Appl. Chem. 2020, 11, 10716–10728.
- Hemmati, S.; Kamangar, S.A.; Ahmeda, A.; Zangeneh, M.M.; Zangeneh, A. Application of copper nanoparticles containing natural compounds in the treatment of bacterial and fungal diseases. Appl. Organomet. Chem. 2020, 34, e5465.
- Tyagi, P.K.; Arya, A.; Mazumder, A.M.; Tyagi, S. Development of copper nanoparticles and their prospective uses as antioxidants, antimicrobials, anticancer agents in the pharmaceutical sector. Precis. Nanomed. 2023, 6, 1048–1065.
- Jagaran, K.; Singh, M. Copolymer-Green-Synthesized Copper Oxide Nanoparticles Enhance Folate-Targeting in Cervical Cancer Cells In Vitro. Polymers 2023, 15, 2393.
- Kang, X.; Wang, J.; Huang, C.-H.; Wibowo, F.S.; Amin, R.; Chen, P.; Li, F. Diethyldithiocarbamate copper nanoparticle overcomes resistance in cancer therapy without inhibiting P-glycoprotein. Nanomed. Nanotechnol. Biol. Med. 2023, 47, 102620.
- Woźniak-Budych, M.J.; Przysiecka, Ł.; Maciejewska, B.M.; Wieczorek, D.; Staszak, K.; Jarek, M.; Jesionowski, T.; Jurga, S. Facile Synthesis of Sulfobetaine-Stabilized Cu2O Nanoparticles and Their Biomedical Potential. ACS Biomater. Sci. Eng. 2017, 3, 3183–3194.
- Woźniak-Budych, M.J.; Langer, K.; Peplińska, B.; Przysiecka, Ł.; Jarek, M.; Jarzębski, M.; Jurga, S. Copper-gold nanoparticles: Fabrication, characteristic and application as drug carriers. Mater. Chem. Phys. 2016, 179, 242–253.
- Zughaibi, T.A.; Mirza, A.A.; Suhail, M.; Jabir, N.R.; Zaidi, S.K.; Wasi, S.; Zawawi, A.; Tabrez, S. Evaluation of Anticancer Potential of Biogenic Copper Oxide Nanoparticles (CuO NPs) against Breast Cancer. J. Nanomater. 2022, 2022, 5326355.
- Mahmood, R.I.; Kadhim, A.A.; Ibraheem, S.; Albukhaty, S.; Mohammed-Salih, H.S.; Abbas, R.H.; Jabir, M.S.; Mohammed, M.K.A.; Nayef, U.M.; AlMalki, F.A.; et al. Biosynthesis of copper oxide nanoparticles mediated Annona muricata as cytotoxic and apoptosis inducer factor in breast cancer cell lines. Sci. Rep. 2022, 12, 16165.
- Chen, H.; Feng, X.; Gao, L.; Mickymaray, S.; Paramasivam, A.; Alfaiz, F.A.; Almasmoum, H.A.; Ghaith, M.M.; Almaimani, R.A.; Ibrahim, I.A.A. Inhibiting the PI3K/AKT/mTOR signalling pathway with copper oxide nanoparticles from Houttuynia cordata plant: Attenuating the proliferation of cervical cancer cells. Artif. Cells Nanomed. Biotechnol. 2021, 49, 240–249.
- Phull, A.-R.; Ali, A.; Dhong, K.R.; Zia, M.; Mahajan, P.G.; Park, H.-J. Synthesis, characterization, anticancer activity assessment and apoptosis signaling of fucoidan mediated copper oxide nanoparticles. Arab. J. Chem. 2021, 14, 103250.
- Abdelhakm, L.O.; Kandil, E.I.; Mansour, S.Z.; El-Sonbaty, S.M. Chrysin Encapsulated Copper Nanoparticles with Low Dose of Gamma Radiation Elicit Tumor Cell Death Through p38 MAPK/NF-κB Pathways. Biol. Trace Element Res. 2023, 1–20.
- Benguigui, M.; Weitz, I.S.; Timaner, M.; Kan, T.; Shechter, D.; Perlman, O.; Sivan, S.; Raviv, Z.; Azhari, H.; Shaked, Y. Copper oxide nanoparticles inhibit pancreatic tumor growth primarily by targeting tumor initiating cells. Sci. Rep. 2019, 9, 12613.
- Yuan, Z.; Qu, S.; He, Y.; Xu, Y.; Liang, L.; Zhou, X.; Gui, L.; Gu, Y.; Chen, H. Thermosensitive drug-loading system based on copper sulfide nanoparticles for combined photothermal therapy and chemotherapy in vivo. Biomater. Sci. 2018, 6, 3219–3230.
This entry is offline, you can click here to edit this entry!
