Tuberculosis (TB) and Human Immunodeficiency Virus (HIV) co-infection continues to pose a significant healthcare burden. HIV co-infection during TB predisposes the host to the reactivation of latent TB infection (LTBI), worsening disease conditions and mortality. There is a lack of biomarkers of LTBI reactivation and/or immune-related transcriptional signatures to distinguish active TB from LTBI and predict TB reactivation upon HIV co-infection. Characterizing individual cells using next-generation sequencing-based technologies has facilitated novel biological discoveries about infectious diseases, including TB and HIV pathogenesis.
- TB/HIV co-infection
- single cell analysis
- latent TB infection
- biomarkers
1. Introduction
2. scRNA-Seq—A Tool to Study Host–Pathogen Interactions and Biosignatures in TB
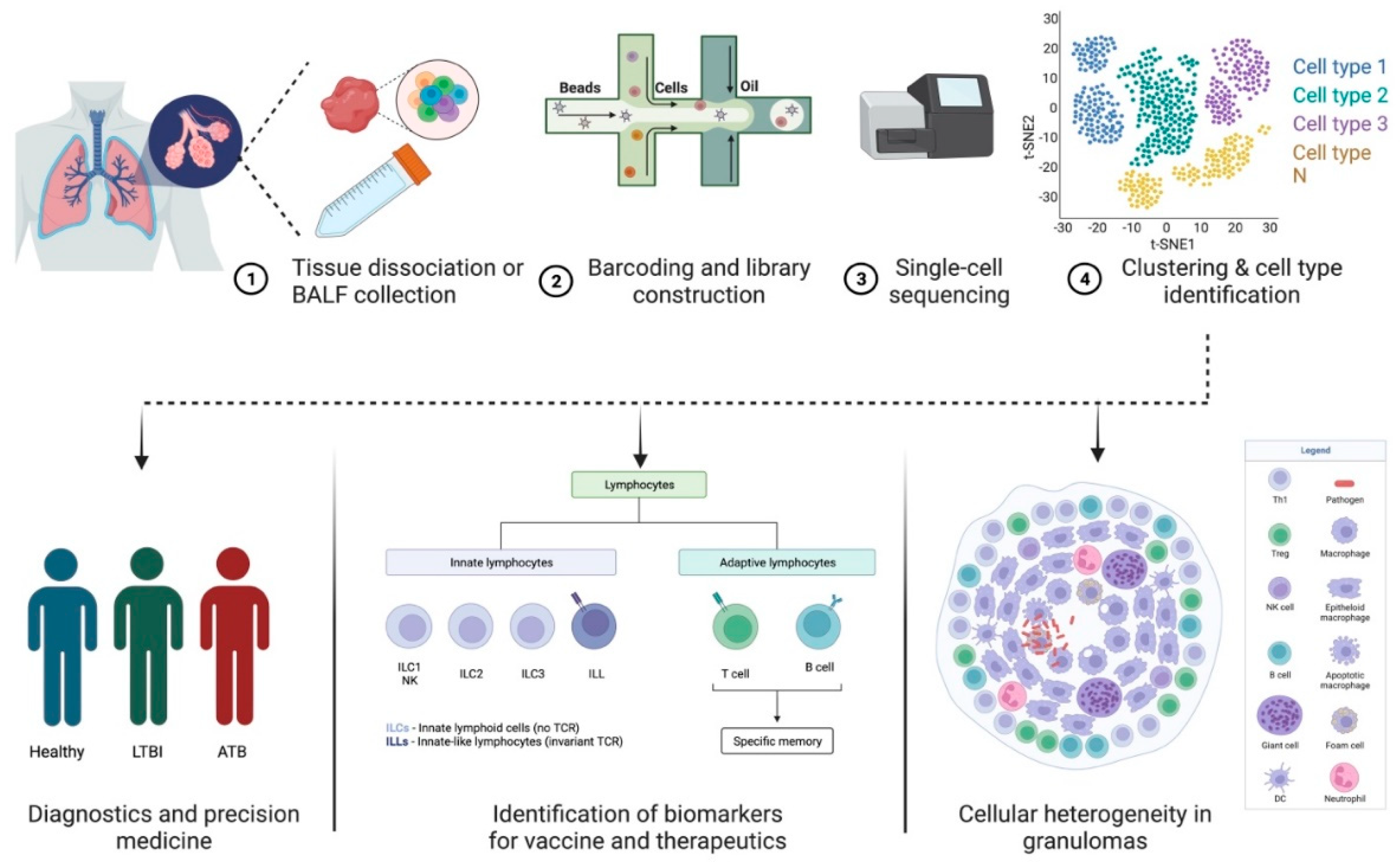
3. Predicting Disease Progression in TB and HIV Using scRNA-Seq
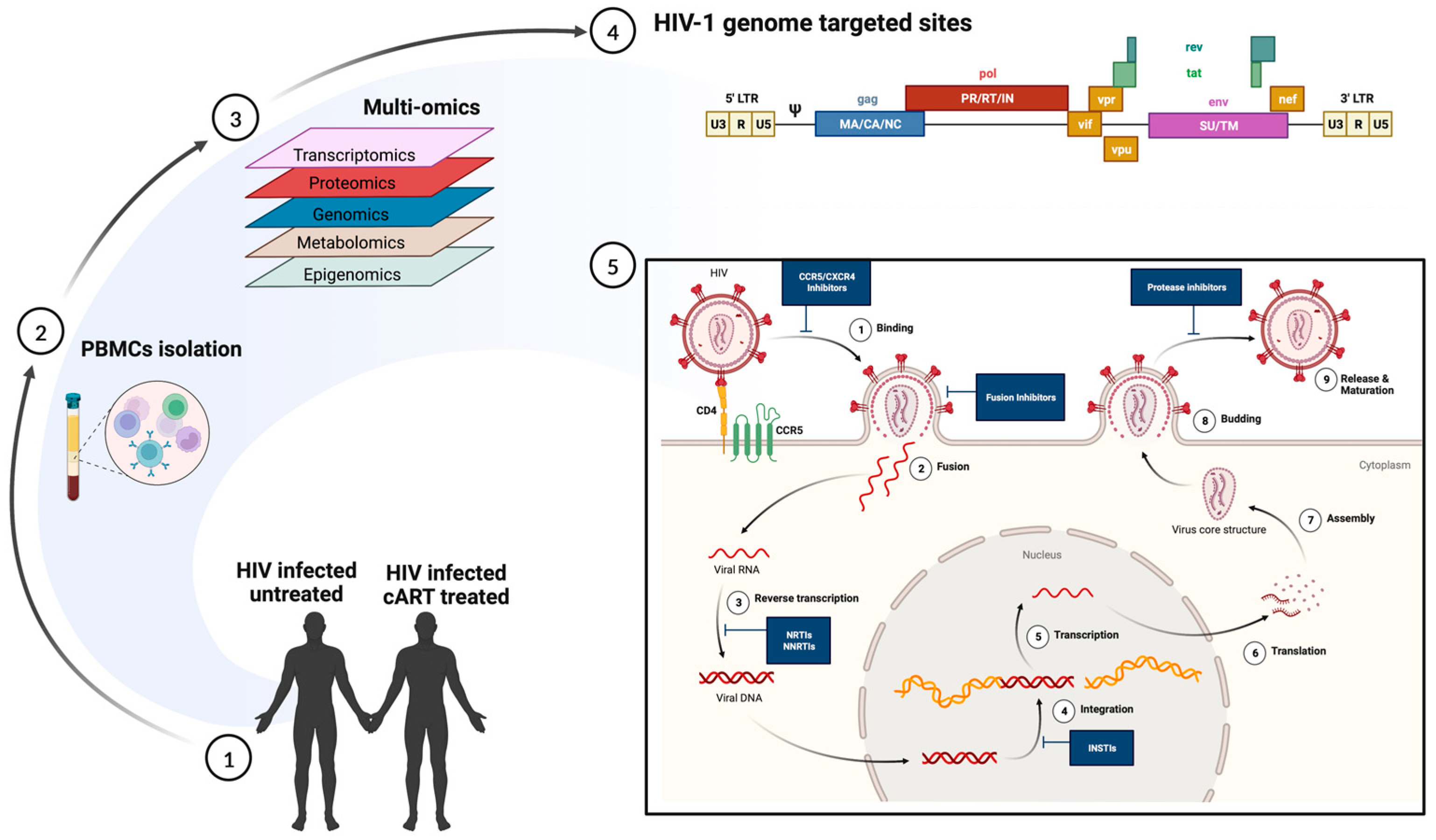
| Study | Technique | Observation | Ref. |
|---|---|---|---|
| Defining HIV-permissive cells | scRNA-seq | TCR stimulation mainly contributes to transcriptional heterogeneity in CD4+ T cells, which regulates HIV transcription. | [53] |
| Immune responses during hyperacute HIV infection | scRNA-seq on peripheral blood mononuclear cells from four untreated individuals before and longitudinally during acute infection |
Immune cell responses to HIV infection within the first weeks of infection, such as proliferating natural killer cells, which potentially may be associated with viral control | [55] |
| Restoration of T cell function after ART | scRNA-seq on peripheral T cells on chronic HIV-infected treatment-naïve or ART-treated patients | Significant loss of naïve T cells, prolonged inflammation, and increased response to interferon-α in treatment-naïve patients, partially restored by ART. Granulosyn expressing CD4+ and CD8+ Effector cell clusters correlated with poor immune restoration |
[57] |
| Immune Exhaustion in Chronic HIV Infection | scRNA-seq on peripheral blood mononuclear cells from HIV patients and healthy subjects | An inhibitory receptor KLRG1 was identified in an HIV-1 specific exhausted CD8+ T cell population expressing KLRG1, TIGIT, and Tbetdim Eomeshi markers | [58] |
| Immune Reconstitution failure in (HAART)-treated HIV patients | scRNA-seq and scATAC-seq analysis of peripheral blood mononuclear cells (PBMCs) derived from immune non-responder [61] and responder (IR) | Low expression of mucosal-associated invariant T (MAIT) cells in INRs, which exhibited transcriptional profiles associated with impaired mitochondrial function and apoptosis signaling | [59] |
| Cellular transcriptome changes induced by active HIV replication in macrophages | An in vitro system to model HIV-1 infection of macrophages and single-cell RNA sequencing (scRNA-seq) to compare the transcriptomes of uninfected cells, cells harboring pre-integration complexes (PIC), and those containing integrated provirus and making late HIV proteins | NFkB- and AP-1-promoted transcription characterize PIC cell transcriptomes, while E2F family transcription products distinguish transcriptomes of cells transcribing from provirus | [54] |
| Differential virus reactivation potential | Primary CD4+ T-cell model expression HIV green fluorescent protein (GFP); scRNA seq | Global transcriptomic profiles of cells with reactivated HIV showed higher cellular activation and metabolic activity | [62] |
| Characterize latent cells reactivated by a single round of stimulation | Latent cell capture; LURE. Purified CD4+ T cells from peripheral blood of ART-treated patients activated by LRS and sorted based on the expression of HIV-env protein and HIV-gag mRNA | CD4+ T cells harboring proviruses with identical Env sequences also showed identical TCRs. Reservoir cells arise by clonal expansion, and the reservoir is maintained by balanced cell division and cell death. Reactivated latent cells express a distinct transcriptional program that suppresses HIV-1 transcription, which helps them survive | [63] |
| Characterize reactivated cells within 24 h of latency reversal | Sortseq; stimulated peripheral blood CD4+ T cells from ART-treated, virally suppressed patients | HIV+ cells enriched in TH1 phenotype upregulate cellular factors that support HIV transcription and promote cellular survival. HIV promoter drives high aberrant host gene transcription downstream of the integration site | [64] |
| Transcriptome of quiescent reservoir memory CD4+ T cells harboring intact HIV provirus | Quiescent reservoir memory CD4+ T cells enriched using their unique TCR as a molecule, identified using scRNA-seq | HIV–proviral integration and latency did not induce a specific transcriptional program | [65] |
| Characterize HIV reservoir cells after suppressive ART-therapy | ECCITE-seq, which captures surface protein expression, cellular transcriptome, HIV-1 RNA, and TCR sequence within the same single cells in longitudinally archived paired samples during actual viremia and one-year post-ART | HIV revised in heterogenous granzyme B+ Th1 effector memory CD4+ T cells with robust antigen response, proliferation potential, and long-term clonal stability | [66] |
| Isolate reservoir cells from HIV patients treated with long-term ART | FIND-seq, simultaneous capture of polyadenylated RNA and HIV DNA from single cells, and scRNA-seq without latency reversal | HIV-DNA+ memory CD4+ T cells inhibited death receptor, necroptosis, and antiproliferative signaling but showed higher expression of known negative regulators of HIV transcription, thus favoring long-term survival of the cells and HIV silencing | [67] |
| The transcriptome of HIV-infected and HIV-exposed peripheral blood monocytes | scRNA seq | Monocytes provide varying degrees of permissive cellular environment for HIV infection, and ART has a detrimental effect on monocyte function | [68] |
| scRNA profiling of HIV-infected cells in CNS | Single nucleus RNA-seq of archived brain tissue | HIV-induced interferon response modulates host chromatin conformation and HIV integration sites in microglia | [69] |
4. Implications of scRNA-Seq in Animal Models of TB and HIV
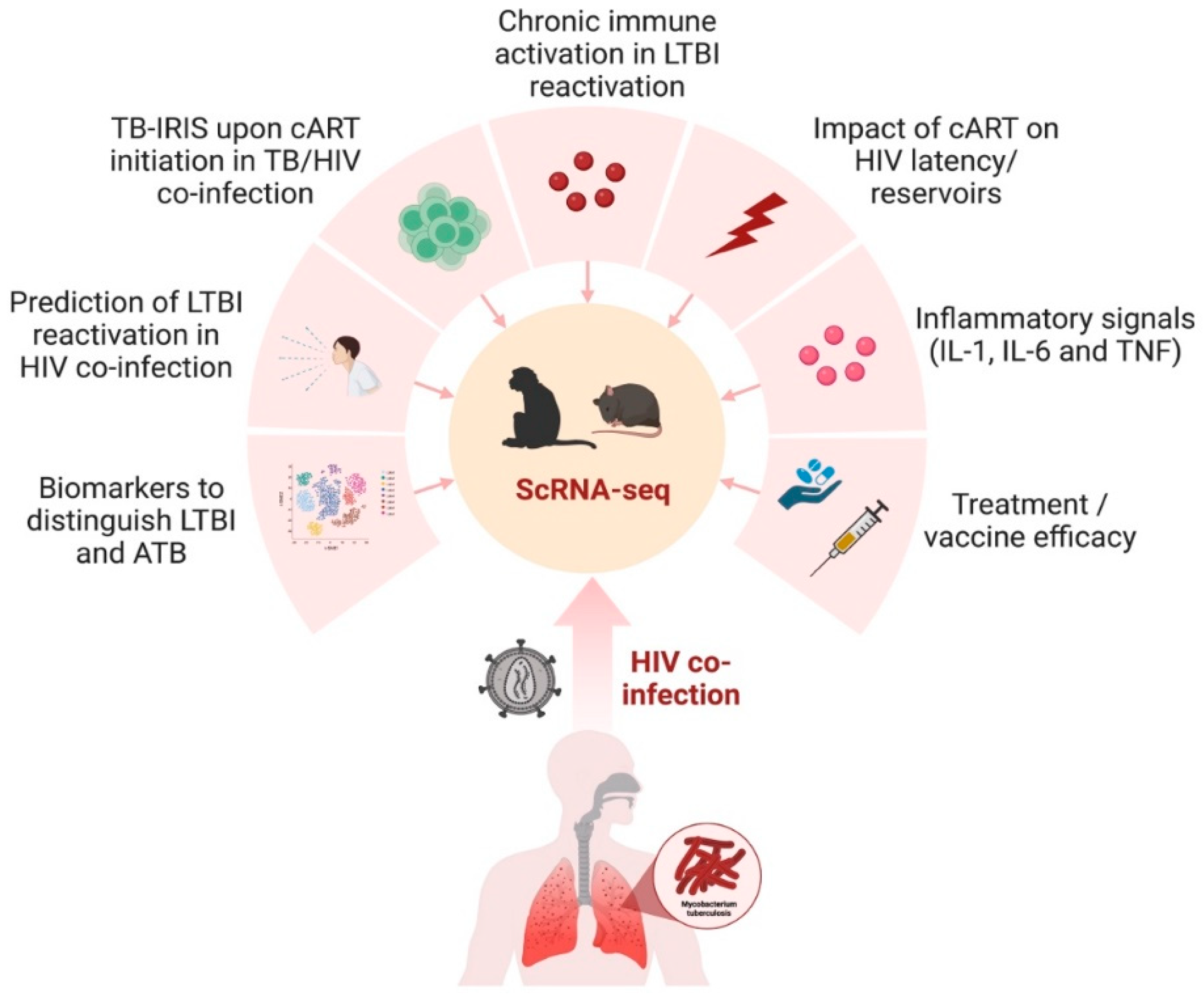
This entry is adapted from the peer-reviewed paper 10.3390/cells12182295
References
- Sharan, R.; Bucşan, A.N.; Ganatra, S.; Paiardini, M.; Mohan, M.; Mehra, S.; Khader, S.A.; Kaushal, D. Chronic Immune Activation in TB/HIV Co-infection. Trends Microbiol. 2020, 28, 619–632.
- Jones-López, E.C.; Namugga, O.; Mumbowa, F.; Ssebidandi, M.; Mbabazi, O.; Moine, S.; Mboowa, G.; Fox, M.P.; Reilly, N.; Ayakaka, I.; et al. Cough aerosols of Mycobacterium tuberculosis predict new infection: A household contact study. Am. J. Respir. Crit. Care Med. 2013, 187, 1007–1015.
- World Health Organization. Global Tuberculosis Report 2022. 2022. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed on 29 May 2023).
- Adhikari, N.; Bhattarai, R.B.; Basnet, R.; Joshi, L.R.; Tinkari, B.S.; Thapa, A.; Joshi, B. Prevalence and associated risk factors for tuberculosis among people living with HIV in Nepal. PLoS ONE 2022, 17, e0262720.
- Runels, T.; Ragan, E.J.; Ventura, A.S.; Winter, M.R.; White, L.F.; Horsburgh, C.R.; Samet, J.H.; Saitz, R.; Jacobson, K.R. Testing and treatment for latent tuberculosis infection in people living with HIV and substance dependence: A prospective cohort study. BMJ Open 2022, 12, e058751.
- Shea, K.M.; Kammerer, J.S.; Winston, C.A.; Navin, T.R.; Horsburgh, C.R., Jr. Estimated rate of reactivation of latent tuberculosis infection in the United States, overall and by population subgroup. Am. J. Epidemiol. 2014, 179, 216–225.
- Bruchfeld, J.; Correia-Neves, M.; Källenius, G. Tuberculosis and HIV Coinfection. Cold Spring Harb. Perspect. Med. 2015, 5, a017871.
- Lavalett, L.; Rodriguez, H.; Ortega, H.; Sadee, W.; Schlesinger, L.S.; Barrera, L.F. Alveolar macrophages from tuberculosis patients display an altered inflammatory gene expression profile. Tuberculosis 2017, 107, 156–167.
- Guo, Q.; Zhong, Y.; Wang, Z.; Cao, T.; Zhang, M.; Zhang, P.; Huang, W.; Bi, J.; Yuan, Y.; Ou, M.; et al. Single-cell transcriptomic landscape identifies the expansion of peripheral blood monocytes as an indicator of HIV-1-TB co-infection. Cell Insight 2022, 1, 100005.
- McCaffrey, E.F.; Donato, M.; Keren, L.; Chen, Z.; Delmastro, A.; Fitzpatrick, M.B.; Gupta, S.; Greenwald, N.F.; Baranski, A.; Graf, W.; et al. The immunoregulatory landscape of human tuberculosis granulomas. Nat. Immunol. 2022, 23, 318–329.
- Tilahun, M.; Shibabaw, A.; Kiflie, A.; Bewket, G.; Abate, E.; Gelaw, B. Latent tuberculosis infection and associated risk factors among people living with HIV and apparently healthy blood donors at the University of Gondar referral hospital, Northwest Ethiopia. BMC Res. Notes 2019, 12, 515.
- Hoerter, A.; Arnett, E.; Schlesinger, L.S.; Pienaar, E. Systems biology approaches to investigate the role of granulomas in TB-HIV coinfection. Front. Immunol. 2022, 13, 1014515.
- Bucşan, A.N.; Chatterjee, A.; Singh, D.K.; Foreman, T.W.; Lee, T.H.; Threeton, B.; Kirkpatrick, M.G.; Ahmed, M.; Golden, N.; Alvarez, X.; et al. Mechanisms of reactivation of latent tuberculosis infection due to SIV coinfection. J. Clin. Investig. 2019, 129, 5254–5260.
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat. Rev. Microbiol. 2018, 16, 80–90.
- Souriant, S.; Balboa, L.; Dupont, M.; Pingris, K.; Kviatcovsky, D.; Cougoule, C.; Lastrucci, C.; Bah, A.; Gasser, R.; Poincloux, R.; et al. Tuberculosis Exacerbates HIV-1 Infection through IL-10/STAT3-Dependent Tunneling Nanotube Formation in Macrophages. Cell Rep. 2019, 26, 3586–3599.
- Wyndham-Thomas, C.; Corbière, V.; Selis, E.; Payen, M.C.; Goffard, J.C.; Van Vooren, J.P.; Mascart, F.; Dirix, V. Immune Activation by Mycobacterium tuberculosis in HIV-Infected and -Uninfected Subjects. J. Acquir. Immune Defic. Syndr. 2017, 74, 103–111.
- LaVergne, S.; Umlauf, A.; McCutchan, A.; Heaton, R.; Benson, C.; Kumarasamy, N.; Bharti, A.R. Impact of Latent Tuberculosis Infection on Neurocognitive Functioning and Inflammation in HIV-Infected and Uninfected South Indians. J. Acquir. Immune Defic. Syndr. 2020, 84, 430–436.
- Sharan, R.; Ganatra, S.R.; Bucsan, A.N.; Cole, J.; Singh, D.K.; Alvarez, X.; Gough, M.; Alvarez, C.; Blakley, A.; Ferdin, J.; et al. Antiretroviral therapy timing impacts latent tuberculosis infection reactivation in a Mycobacterium tuberculosis/SIV coinfection model. J. Clin. Investig. 2022, 132.
- Ganatra, S.R.; Bucşan, A.N.; Alvarez, X.; Kumar, S.; Chatterjee, A.; Quezada, M.; Fish, A.; Singh, D.K.; Singh, B.; Sharan, R.; et al. Antiretroviral therapy does not reduce tuberculosis reactivation in a tuberculosis-HIV coinfection model. J. Clin. Investig. 2020, 130, 5171–5179.
- Teklu, A.M.; Nega, A.; Mamuye, A.T.; Sitotaw, Y.; Kassa, D.; Mesfin, G.; Belayihun, B.; Medhin, G.; Yirdaw, K. Factors Associated with Mortality of TB/HIV Co-infected Patients in Ethiopia. Ethiop. J. Health Sci. 2017, 27, 29–38.
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200.
- Paiardini, M.; Müller-Trutwin, M. HIV-associated chronic immune activation. Immunol. Rev. 2013, 254, 78–101.
- Wilkinson, K.A.; Meintjes, G.; Seldon, R.; Goliath, R.; Wilkinson, R.J. Immunological characterisation of an unmasking TB-IRIS case. S. Afr. Med. J. 2012, 102, 512–517.
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766.
- Gideon, H.P.; Flynn, J.L. Latent tuberculosis: What the host “sees”? Immunol. Res. 2011, 50, 202–212.
- Wong, N.S.; Leung, C.C.; Chan, K.C.W.; Chan, W.K.; Lin, A.W.C.; Lee, S.S. A longitudinal study on latent TB infection screening and its association with TB incidence in HIV patients. Sci. Rep. 2019, 9, 10093.
- Liao, S.Y.; Atif, S.M.; Mould, K.; Konigsberg, I.R.; Fu, R.; Davidson, E.; Li, L.; Fontenot, A.P.; Maier, L.A.; Yang, I.V. Single-cell RNA sequencing identifies macrophage transcriptional heterogeneities in granulomatous diseases. Eur. Respir. J. 2021, 57, 2003794.
- Avraham, R.; Haseley, N.; Brown, D.; Penaranda, C.; Jijon, H.B.; Trombetta, J.J.; Satija, R.; Shalek, A.K.; Xavier, R.J.; Regev, A.; et al. Pathogen Cell-to-Cell Variability Drives Heterogeneity in Host Immune Responses. Cell 2015, 162, 1309–1321.
- Avraham, R.; Hung, D.T. A perspective on single cell behavior during infection. Gut Microbes 2016, 7, 518–525.
- Gideon, H.P.; Hughes, T.K.; Tzouanas, C.N.; Wadsworth, M.H., 2nd; Tu, A.A.; Gierahn, T.M.; Peters, J.M.; Hopkins, F.F.; Wei, J.R.; Kummerlowe, C.; et al. Multimodal profiling of lung granulomas in macaques reveals cellular correlates of tuberculosis control. Immunity 2022, 55, 827–846.
- Ma, F.; Hughes, T.K.; Teles, R.M.B.; Andrade, P.R.; de Andrade Silva, B.J.; Plazyo, O.; Tsoi, L.C.; Do, T.; Wadsworth, M.H., 2nd; Oulee, A.; et al. The cellular architecture of the antimicrobial response network in human leprosy granulomas. Nat. Immunol. 2021, 22, 839–850.
- Cai, Y.; Dai, Y.; Wang, Y.; Yang, Q.; Guo, J.; Wei, C.; Chen, W.; Huang, H.; Zhu, J.; Zhang, C.; et al. Single-cell transcriptomics of blood reveals a natural killer cell subset depletion in tuberculosis. EBioMedicine 2020, 53, 102686.
- Xu, Y.; Tan, Y.; Zhang, X.; Cheng, M.; Hu, J.; Liu, J.; Chen, X.; Zhu, J. Comprehensive identification of immuno-related transcriptional signature for active pulmonary tuberculosis by integrated analysis of array and single cell RNA-seq. J. Infect. 2022, 85, 534–544.
- Kulkarni, V.; Queiroz, A.T.L.; Sangle, S.; Kagal, A.; Salvi, S.; Gupta, A.; Ellner, J.; Kadam, D.; Rolla, V.C.; Andrade, B.B.; et al. A Two-Gene Signature for Tuberculosis Diagnosis in Persons with Advanced HIV. Front. Immunol. 2021, 12, 631165.
- Hillman, H.; Khan, N.; Singhania, A.; Dubelko, P.; Soldevila, F.; Tippalagama, R.; DeSilva, A.D.; Gunasena, B.; Perera, J.; Scriba, T.J.; et al. Single-cell profiling reveals distinct subsets of CD14+ monocytes drive blood immune signatures of active tuberculosis. Front. Immunol. 2022, 13, 1087010.
- Akter, S.; Chauhan, K.S.; Dunlap, M.D.; Choreño-Parra, J.A.; Lu, L.; Esaulova, E.; Zúñiga, J.; Artyomov, M.N.; Kaushal, D.; Khader, S.A. Mycobacterium tuberculosis infection drives a type I IFN signature in lung lymphocytes. Cell Rep. 2022, 39, 110983.
- Estévez, O.; Anibarro, L.; Garet, E.; Pallares, Á.; Barcia, L.; Calviño, L.; Maueia, C.; Mussá, T.; Fdez-Riverola, F.; Glez-Peña, D.; et al. An RNA-seq Based Machine Learning Approach Identifies Latent Tuberculosis Patients with an Active Tuberculosis Profile. Front. Immunol. 2020, 11, 1470.
- Nathan, A.; Beynor, J.I.; Baglaenko, Y.; Suliman, S.; Ishigaki, K.; Asgari, S.; Huang, C.C.; Luo, Y.; Zhang, Z.; Lopez, K.; et al. Multimodally profiling memory T cells from a tuberculosis cohort identifies cell state associations with demographics, environment and disease. Nat. Immunol. 2021, 22, 781–793.
- Boisson-Dupuis, S.; Bustamante, J.; El-Baghdadi, J.; Camcioglu, Y.; Parvaneh, N.; El Azbaoui, S.; Agader, A.; Hassani, A.; El Hafidi, N.; Mrani, N.A.; et al. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol. Rev. 2015, 264, 103–120.
- Scriba, T.J.; Kalsdorf, B.; Abrahams, D.A.; Isaacs, F.; Hofmeister, J.; Black, G.; Hassan, H.Y.; Wilkinson, R.J.; Walzl, G.; Gelderbloem, S.J.; et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 2008, 180, 1962–1970.
- Okada, S.; Markle, J.G.; Deenick, E.K.; Mele, F.; Averbuch, D.; Lagos, M.; Alzahrani, M.; Al-Muhsen, S.; Halwani, R.; Ma, C.S.; et al. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 2015, 349, 606–613.
- Bossel Ben-Moshe, N.; Hen-Avivi, S.; Levitin, N.; Yehezkel, D.; Oosting, M.; Joosten, L.A.B.; Netea, M.G.; Avraham, R. Predicting bacterial infection outcomes using single cell RNA-sequencing analysis of human immune cells. Nat. Commun. 2019, 10, 3266.
- Oelen, R.; de Vries, D.H.; Brugge, H.; Gordon, M.G.; Vochteloo, M.; Ye, C.J.; Westra, H.J.; Franke, L.; van der Wijst, M.G.P. Single-cell RNA-sequencing of peripheral blood mononuclear cells reveals widespread, context-specific gene expression regulation upon pathogenic exposure. Nat. Commun. 2022, 13, 3267.
- Thobakgale, C.; Naidoo, K.; McKinnon, L.R.; Werner, L.; Samsunder, N.; Karim, S.A.; Ndung’u, T.; Altfeld, M.; Naidoo, K. Interleukin 1-Beta (IL-1β) Production by Innate Cells Following TLR Stimulation Correlates with TB Recurrence in ART-Treated HIV-Infected Patients. J. Acquir. Immune Defic. Syndr. 2017, 74, 213–220.
- McLaren, P.J.; Fellay, J. HIV-1 and human genetic variation. Nat. Rev. Genet. 2021, 22, 645–657.
- Horton, R.E.; McLaren, P.J.; Fowke, K.; Kimani, J.; Ball, T.B. Cohorts for the study of HIV-1-exposed but uninfected individuals: Benefits and limitations. J. Infect. Dis. 2010, 202 (Suppl. S3), S377–S381.
- Kulkarni, P.S.; Butera, S.T.; Duerr, A.C. Resistance to HIV-1 infection: Lessons learned from studies of highly exposed persistently seronegative (HEPS) individuals. AIDS Rev. 2003, 5, 87–103.
- Sabin, C.A.; Lundgren, J.D. The natural history of HIV infection. Curr. Opin. HIV AIDS 2013, 8, 311–317.
- Pollara, J.; Khanal, S.; Edwards, R.W.; Hora, B.; Ferrari, G.; Haynes, B.F.; Bradley, T. Single-cell analysis of immune cell transcriptome during HIV-1 infection and therapy. BMC Immunol. 2022, 23, 48.
- Ciuffi, A.; Bleiber, G.; Munoz, M.; Martinez, R.; Loeuillet, C.; Rehr, M.; Fischer, M.; Gunthard, H.F.; Oxenius, A.; Meylan, P.; et al. Entry and transcription as key determinants of differences in CD4 T-cell permissiveness to human immunodeficiency virus type 1 infection. J. Virol. 2004, 78, 10747–10754.
- Mohammadi, P.; Desfarges, S.; Bartha, I.; Joos, B.; Zangger, N.; Munoz, M.; Gunthard, H.F.; Beerenwinkel, N.; Telenti, A.; Ciuffi, A. 24 hours in the life of HIV-1 in a T cell line. PLoS Pathog. 2013, 9, e1003161.
- Rausell, A.; Munoz, M.; Martinez, R.; Roger, T.; Telenti, A.; Ciuffi, A. Innate immune defects in HIV permissive cell lines. Retrovirology 2016, 13, 43.
- Rato, S.; Rausell, A.; Munoz, M.; Telenti, A.; Ciuffi, A. Single-cell analysis identifies cellular markers of the HIV permissive cell. PLoS Pathog. 2017, 13, e1006678.
- Lim, A.L.; Moos, P.; Pond, C.D.; Larson, E.C.; Martins, L.J.; Szaniawski, M.A.; Planelles, V.; Barrows, L.R. HIV-1 provirus transcription and translation in macrophages differs from pre-integrated cDNA complexes and requires E2F transcriptional programs. Virulence 2022, 13, 386–413.
- Kazer, S.W.; Aicher, T.P.; Muema, D.M.; Carroll, S.L.; Ordovas-Montanes, J.; Miao, V.N.; Tu, A.A.; Ziegler, C.G.K.; Nyquist, S.K.; Wong, E.B.; et al. Integrated single-cell analysis of multicellular immune dynamics during hyperacute HIV-1 infection. Nat. Med. 2020, 26, 511–518.
- Mahnke, Y.D.; Fletez-Brant, K.; Sereti, I.; Roederer, M. Reconstitution of Peripheral T Cells by Tissue-Derived CCR4+ Central Memory Cells Following HIV-1 Antiretroviral Therapy. Pathog. Immun. 2016, 1, 260–290.
- Wang, X.M.; Zhang, J.Y.; Xing, X.; Huang, H.H.; Xia, P.; Dai, X.P.; Hu, W.; Zhang, C.; Song, J.W.; Fan, X.; et al. Global transcriptomic characterization of T cells in individuals with chronic HIV-1 infection. Cell Discov. 2022, 8, 29.
- Wang, S.; Zhang, Q.; Hui, H.; Agrawal, K.; Karris, M.A.Y.; Rana, T.M. An atlas of immune cell exhaustion in HIV-infected individuals revealed by single-cell transcriptomics. Emerg. Microbes Infect. 2020, 9, 2333–2347.
- Li, H.; Tang, Y.; Wang, Y.; Li, Y.; Yang, Y.; Liao, K.; Song, F.; Deng, S.; Chen, Y. Single-cell sequencing resolves the landscape of immune cells and regulatory mechanisms in HIV-infected immune non-responders. Cell Death Dis. 2022, 13, 849.
- Niu, M.; Morsey, B.; Lamberty, B.G.; Emanuel, K.; Yu, F.; Leon-Rivera, R.; Berman, J.W.; Gaskill, P.J.; Matt, S.M.; Ciborowski, P.S.; et al. Methamphetamine Increases the Proportion of SIV-Infected Microglia/Macrophages, Alters Metabolic Pathways, and Elevates Cell Death Pathways: A Single-Cell Analysis. Viruses 2020, 12, 1297.
- Tiwari, S.; van Tonder, A.J.; Vilchèze, C.; Mendes, V.; Thomas, S.E.; Malek, A.; Chen, B.; Chen, M.; Kim, J.; Blundell, T.L.; et al. Arginine-deprivation-induced oxidative damage sterilizes Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2018, 115, 9779–9784.
- Golumbeanu, M.; Cristinelli, S.; Rato, S.; Munoz, M.; Cavassini, M.; Beerenwinkel, N.; Ciuffi, A. Single-Cell RNA-Seq Reveals Transcriptional Heterogeneity in Latent and Reactivated HIV-Infected Cells. Cell Rep. 2018, 23, 942–950.
- Cohn, L.B.; da Silva, I.T.; Valieris, R.; Huang, A.S.; Lorenzi, J.C.C.; Cohen, Y.Z.; Pai, J.A.; Butler, A.L.; Caskey, M.; Jankovic, M.; et al. Clonal CD4+ T cells in the HIV-1 latent reservoir display a distinct gene profile upon reactivation. Nat. Med. 2018, 24, 604–609.
- Liu, R.; Yeh, Y.J.; Varabyou, A.; Collora, J.A.; Sherrill-Mix, S.; Talbot, C.C., Jr.; Mehta, S.; Albrecht, K.; Hao, H.; Zhang, H.; et al. Single-cell transcriptional landscapes reveal HIV-1-driven aberrant host gene transcription as a potential therapeutic target. Sci. Transl. Med. 2020, 12, eaaz0802.
- Weymar, G.H.J.; Bar-On, Y.; Oliveira, T.Y.; Gaebler, C.; Ramos, V.; Hartweger, H.; Breton, G.; Caskey, M.; Cohn, L.B.; Jankovic, M.; et al. Distinct gene expression by expanded clones of quiescent memory CD4+ T cells harboring intact latent HIV-1 proviruses. Cell Rep. 2022, 40, 111311.
- Collora, J.A.; Liu, R.; Pinto-Santini, D.; Ravindra, N.; Ganoza, C.; Lama, J.R.; Alfaro, R.; Chiarella, J.; Spudich, S.; Mounzer, K.; et al. Single-cell multiomics reveals persistence of HIV-1 in expanded cytotoxic T cell clones. Immunity 2022, 55, 1013–1031.e7.
- Clark, I.C.; Mudvari, P.; Thaploo, S.; Smith, S.; Abu-Laban, M.; Hamouda, M.; Theberge, M.; Shah, S.; Ko, S.H.; Perez, L.; et al. HIV silencing and cell survival signatures in infected T cell reservoirs. Nature 2023, 614, 318–325.
- Leon-Rivera, R.; Morsey, B.; Niu, M.; Fox, H.S.; Berman, J.W. Interactions of Monocytes, HIV, and ART Identified by an Innovative scRNAseq Pipeline: Pathways to Reservoirs and HIV-Associated Comorbidities. mBio 2020, 11.
- Plaza-Jennings, A.L.; Valada, A.; O’Shea, C.; Iskhakova, M.; Hu, B.; Javidfar, B.; Ben Hutta, G.; Lambert, T.Y.; Murray, J.; Kassim, B.; et al. HIV integration in the human brain is linked to microglial activation and 3D genome remodeling. Mol. Cell 2022, 82, 4647–4663.e8.
- Farhadian, S.F.; Mehta, S.S.; Zografou, C.; Robertson, K.; Price, R.W.; Pappalardo, J.; Chiarella, J.; Hafler, D.A.; Spudich, S.S. Single-cell RNA sequencing reveals microglia-like cells in cerebrospinal fluid during virologically suppressed HIV. JCI Insight 2018, 3, e121718.
- Foreman, T.W.; Mehra, S.; Lackner, A.A.; Kaushal, D. Translational Research in the Nonhuman Primate Model of Tuberculosis. ILAR J. 2017, 58, 151–159.
- Scanga, C.A.; Flynn, J.L. Modeling tuberculosis in nonhuman primates. Cold Spring Harb. Perspect. Med. 2014, 4, a018564.
- Cooper, E.B.; Brent, L.J.N.; Snyder-Mackler, N.; Singh, M.; Sengupta, A.; Khatiwada, S.; Malaivijitnond, S.; Qi Hai, Z.; Higham, J.P. The rhesus macaque as a success story of the Anthropocene. Elife 2022, 11, e78169.
- Sharan, R.; Ganatra, S.R.; Singh, D.K.; Cole, J.; Foreman, T.W.; Thippeshappa, R.; Peloquin, C.A.; Shivanna, V.; Gonzalez, O.; Day, C.L.; et al. Isoniazid and rifapentine treatment effectively reduces persistent M. tuberculosis infection in macaque lungs. J. Clin. Investig. 2022, 132, e121718.
- Esaulova, E.; Das, S.; Singh, D.K.; Choreño-Parra, J.A.; Swain, A.; Arthur, L.; Rangel-Moreno, J.; Ahmed, M.; Singh, B.; Gupta, A.; et al. The immune landscape in tuberculosis reveals populations linked to disease and latency. Cell Host Microbe 2021, 29, 165–178.
- Han, L.; Wei, X.; Liu, C.; Volpe, G.; Zhuang, Z.; Zou, X.; Wang, Z.; Pan, T.; Yuan, Y.; Zhang, X.; et al. Cell transcriptomic atlas of the non-human primate Macaca fascicularis. Nature 2022, 604, 723–731.
- Qu, J.; Yang, F.; Zhu, T.; Wang, Y.; Fang, W.; Ding, Y.; Zhao, X.; Qi, X.; Xie, Q.; Chen, M.; et al. A reference single-cell regulomic and transcriptomic map of cynomolgus monkeys. Nat. Commun. 2022, 13, 4069.
- Pisu, D.; Huang, L.; Narang, V.; Theriault, M.; Lê-Bury, G.; Lee, B.; Lakudzala, A.E.; Mzinza, D.T.; Mhango, D.V.; Mitini-Nkhoma, S.C.; et al. Single cell analysis of M. tuberculosis phenotype and macrophage lineages in the infected lung. J. Exp. Med. 2021, 218, e20210615.
- Berry, M.P.; Graham, C.M.; McNab, F.W.; Xu, Z.; Bloch, S.A.; Oni, T.; Wilkinson, K.A.; Banchereau, R.; Skinner, J.; Wilkinson, R.J.; et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 2010, 466, 973–977.
- Pan, J.; Zhang, X.; Xu, J.; Chang, Z.; Xin, Z.; Wang, G. Landscape of Exhausted T Cells in Tuberculosis Revealed by Single-Cell Sequencing. Microbiol. Spectr. 2023, 11, e02839-22.
- Sannier, G.; Dubé, M.; Kaufmann, D.E. Single-Cell Technologies Applied to HIV-1 Research: Reaching Maturity. Front. Microbiol. 2020, 11, 297.
- Mbonye, U.; Karn, J. The Molecular Basis for Human Immunodeficiency Virus Latency. Annu. Rev. Virol. 2017, 4, 261–285.
- Trease, A.J.; Niu, M.; Morsey, B.; Guda, C.; Byrareddy, S.N.; Buch, S.; Fox, H.S. Antiretroviral therapy restores the homeostatic state of microglia in SIV-infected rhesus macaques. J. Leukoc. Biol. 2022, 112, 969–981.
- Han, Q.; Bradley, T.; Williams, W.B.; Cain, D.W.; Montefiori, D.C.; Saunders, K.O.; Parks, R.J.; Edwards, R.W.; Ferrari, G.; Mueller, O.; et al. Neonatal Rhesus Macaques Have Distinct Immune Cell Transcriptional Profiles following HIV Envelope Immunization. Cell Rep. 2020, 30, 1553–1569.
- Marsden, M.D. Benefits and limitations of humanized mice in HIV persistence studies. Retrovirology 2020, 17, 7.
- Victor Garcia, J. Humanized mice for HIV and AIDS research. Curr. Opin. Virol. 2016, 19, 56–64.
- Cheng, L.; Yu, H.; Wrobel, J.A.; Li, G.; Liu, P.; Hu, Z.; Xu, X.N.; Su, L. Identification of pathogenic TRAIL-expressing innate immune cells during HIV-1 infection in humanized mice by scRNA-Seq. JCI Insight 2020, 5, e135344.
- Aso, H.; Nagaoka, S.; Kawakami, E.; Ito, J.; Islam, S.; Tan, B.J.Y.; Nakaoka, S.; Ashizaki, K.; Shiroguchi, K.; Suzuki, Y.; et al. Multiomics Investigation Revealing the Characteristics of HIV-1-Infected Cells In Vivo. Cell Rep. 2020, 32, 107887.
- Nusbaum, R.J.; Calderon, V.E.; Huante, M.B.; Sutjita, P.; Vijayakumar, S.; Lancaster, K.L.; Hunter, R.L.; Actor, J.K.; Cirillo, J.D.; Aronson, J.; et al. Pulmonary Tuberculosis in Humanized Mice Infected with HIV-1. Sci. Rep. 2016, 6, 21522.
- Huante, M.B.; Saito, T.B.; Nusbaum, R.J.; Naqvi, K.F.; Chauhan, S.; Hunter, R.L.; Actor, J.K.; Rudra, J.S.; Endsley, M.A.; Lisinicchia, J.G.; et al. Small Animal Model of Post-chemotherapy Tuberculosis Relapse in the Setting of HIV Co-infection. Front. Cell. Infect. Microbiol. 2020, 10, 150.
- Endsley, J.J.; Huante, M.B.; Naqvi, K.F.; Gelman, B.B.; Endsley, M.A. Advancing our understanding of HIV co-infections and neurological disease using the humanized mouse. Retrovirology 2021, 18, 14.
