Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
The DNA damage response (DDR) is recognized as having an important role in cancer growth and treatment. ATR (ataxia telangiectasia mutated and Rad3-related) kinase, a major regulator of DDR, has shown significant therapeutic potential in cancer treatment. ATR inhibitors have shown anti-tumor effectiveness, not just as monotherapies but also in enhancing the effects of standard chemotherapy, radiation, and immunotherapy.
- ATR
- DNA damage responses
- DNA damage checkpoint signaling
- embryogenesis
- tumorigenesis
- apoptosis
- cancer therapeutics
1. Introduction
Most malignancies have genomic instability, which can cause oncogenesis. In response to DNA damage, cells have evolved several methods to protect their genome. These include the checkpoint mechanism, which protects the genome by limiting cell cycle progression in the event of DNA damage. ATR (ATM and Rad3-related), a member of the PIKK (phosphatidyl inositol 3′ kinase-related kinases) protein family, is a crucial protein in checkpoint responses. ATR is activated by both DNA-damaging agents and halted replication forks. The formation of DNA damage-induced elongation replication protein A (RPA)-coated single-stranded DNA (RPA-ssDNA) during replication stressors and during DNA repair is a common theme that leads to ATR activation [1,2]. ATR interacts with a nuclear partner, ATRIP, to form the ATR–ATRIP complex, which is recruited to the sites of DNA damage by RPA-ssDNA [3,4]. The recruitment of the ATR–ATRIP complex (ATR–ATRIP) to RPA-ssDNA, on the other hand, is insufficient for ATR activation. ATR is autophosphorylated at its T1989 residue [5] after being recruited to DNA damage sites. TopBP1 (topoisomerase 2 binding protein 1) binds at this phosphorylated residue and in turn increases ATR kinase activity [6]. At dsDNA-ssDNA junctions, TopBP1 appears to interact with the Rad9–Rad1–Hus1 (9-1-1) complex [7,8,9]. TopBP1 then directly stimulates the ATR–ATRIP kinase [7,8,10]. The RPA binding protein, Ewing’s tumor-associated antigen 1 (ETAA1), interacts with RPA and functions at stalled replication forks [11,12]. ETAA1, like TopBP1, directly activates ATR–ATRIP [11,12]. In the case of severe damage, activated ATR also stimulates the activities of several key downstream proteins, e.g., p53 and other checkpoint kinases such as Chk1, resulting in an S phase cell cycle interruption to repair DNA damage or apoptosis [2,5,7,8,12,13,14,15,16,17] (Figure 1). The substrates and effectors of ATR and Chk1 have been identified to be a growing collection of DNA replication, repair, or cell cycle proteins. In particular, Chk1 phosphorylation of Cdc25 phosphatases is relevant to DNA damage inducing cancer cell cycle arrest [18]. To correct DNA replication in cells exposed to replication stress, the phosphorylation by ATR of WRN, SMARCAL1, and FANCI is essential [19,20,21]. ATR is also involved in the regulation of numerous DNA repair mechanisms, including homologous recombination, inter-strand crosslink repair, and nucleotide excision repair [22,23,24,25,26].
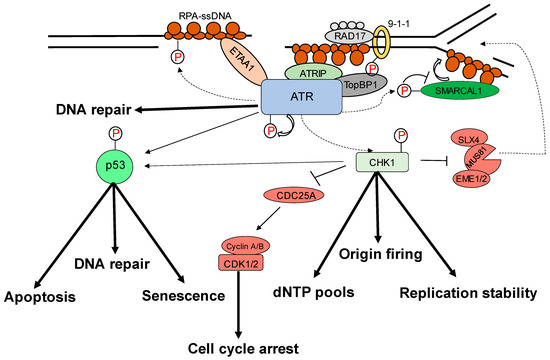
Figure 1. Replication stress-induced ATR–CHK1 activation. ATR is activated by RPA-coated single-stranded DNA (ssDNA) that forms at a stalled replication fork or resected DNA double-strand break (DSB), particularly at the ssDNA/dsDNA confluence. ATR-interacting protein (ATRIP) recruitment results in ATR and RPA-ssDNA complex recognition. It then integrates Rad9–Rad1–hus1 (9-1-1) and DNA topoisomerase 2-binding protein 1 (TOPBP1), activating ATR. ATR phosphorylates checkpoint kinase 1 (CHK1) via the adaptor protein claspin. CHK1 activation can help to prevent genomic instability. The processes promote or prevent the initiation of DNA replication (origin firing), ensure a sufficient supply of deoxynucleotides (dNTPs), stabilize the replication fork, and repair DNA. The signal is sent by transducer proteins to effector proteins such as p53, which is phosphorylated by ATR and CHK1. Cell-cycle arrest, DNA repair, apoptosis, and senescence are all mediated by p53.
2. Role of ATR–Chk1 Pathway in Oncogenesis
In mouse models, homozygous ATR deletion is embryonically lethal, and in mouse embryonic fibroblasts that are acutely genetically inactivated by ATR, one or two rounds of DNA replication are required to leave the cell cycle permanently [4,28]. In contrast, a mouse model expressing kinase-dead ATR (ATR+/KD) but not ATR loss (ATR−/−) exhibited ssDNA-dependent defects at the non-homologous region of X–Y chromosomes during male meiosis, resulting in sterility, and at telomeres, rDNA, and fragile sites during mitosis, resulting in lymphocytopenia [29]. Prompt ATR exchange at DNA damage sites requires ATR kinase activity, resulting in enhancement of Chk1 phosphorylation. ATR-KD traps a fraction of ATR and RPA to chromatin, where ATM/DNA-PKcs hyperphosphorylate RPA and prevent subsequent repair [29]. Because of the replication stress resulting from spontaneously defective DNA, and particularly hard to replicate areas in the genome such as fragile regions, an ATR pathway is essential. Furthermore, genetic inactivation of ATR in adult mice causes premature aging, abnormalities in tissue homeostasis, and progenitor cell depletion in high proliferative regions [30,31].
ATR, on the other hand, is more critical in many tumor cells than it is in normal ones. First, oncoproteins such as the Ras isoforms, Myc, and Cyclin E induce dysregulated signaling that disrupts normal cell cycle regulation and causes replication stress [34]. The ATR pathway plays an important role in the survival of these tumor cells, and several studies have shown that blocking this pathway has a preferentially lethal effect on cells with high levels of oncogene-induced replication stress [33,35,36,37,38,39,40,41]. Second, deficiency in ATM makes the cells more sensitive to ATR inhibition in cell culture and in animal models [42,43,44], a finding that has led to clinical trials of an ATR inhibitor in tumors characterized by ATM deficiency or ATM mutations. Third, in cell line models, loss of specific DNA repair proteins (e.g., XRCC1, ERCC1) causes tumor cell lines more sensitive to ATR inhibition [45,46,47,48]; however, these findings are yet to be implemented in animal models. Fourth, hypoxic cells, which are typically resistant to chemo- and radiation therapy [49], are sensitive to ATR inhibition [50,51,52], likely because hypoxia causes replication stress [53]. Fifth, tumor cells that rely on the alternative lengthening of telomeres (ALT) pathway are also more sensitive to ATR disruption due to ATR’s role in the homologous recombination reactions that maintain these telomeres [54]. Thus, taken together, these observations indicate that multiple events that drive tumorigenesis may create a synthetic lethality for ATR inhibition such as the finding that PARP inhibition is synthetically lethal in BRCA1/2-deficient tumors [55].
3. Cytoplasmic Functions of ATR
Apart from ATR nuclear function regulated through the kinase domain, the cytoplasmic ATR was discovered to serve a significant antiapoptotic effect directly at the mitochondria, independent of nuclear ATR and kinase activity [63]. The nuclear kinase and cytoplasmic antiapoptotic functions of ATR are carried out independently by two prolyl isomeric forms of ATR, namely, trans- and cis-ATRS428P429 [63]. Both of these variants rely on a single-peptide bond orientation in ATR via a prolyl isomerization motif at S428P429 in humans. ATRIP is consistently missing in the cytoplasm, except during its production. In contrast to nuclear ATR, which is constantly in the trans form in a complex with ATRIP, cytoplasmic ATR, which lacks ATRIP, is mostly in the cis isomeric form after DNA damage [63]. Pin1 and PP2A balance the cis and trans cytoplasmic versions of ATR, with the former facilitating the conversion of cis- to trans-ATR by detecting the phosphorylated Serine 428–Proline 429 motif (pS428-P429) in ATR’s N-terminus [63,64]. Although Pin 1 activity promotes trans-ATR synthesis, DAPKI kinase inactivates it in response to DNA damage. In addition, DNA damage activates PP2A, which dephosphorylates ATR (pS428-P429), not only disabling the S428 phosphorylation-dependent Pin1 activity on ATR but also leading to cis-ATR formation ATR [64,65], as ATR is naturally stable in the cis-isomeric form of ATR [65]. Unlike the trans-ATR isoform, the cis-ATR includes an exposed BH3-like domain, which allows it to connect to the proapoptotic tBid protein at the mitochondria [63,64,65,66,67] (Figure 2). This binding prevents tBid from triggering the Bax–Bak polymerization pathway, which is necessary for the intrinsic apoptotic pathway. From here on, cis-ATR performs an antiapoptotic function, allowing the cell to survive long enough to repair its damaged DNA [64]. It is of importance to investigate whether cis-ATR might be used as a potential target for developing effective novel anticancer drugs.
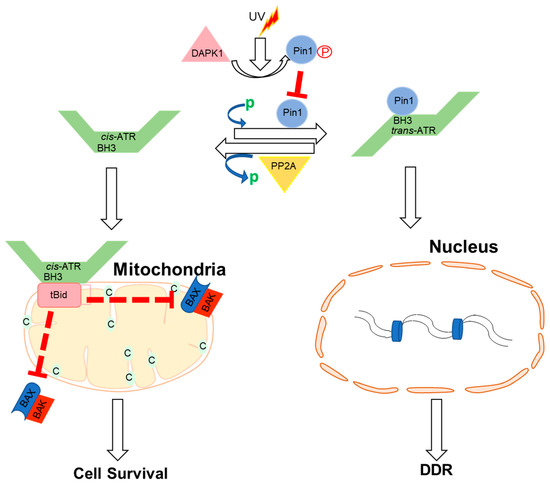
Figure 2. Proposed mechanisms by which ATR plays a direct anti-apoptotic function at the mitochondria. UV irradiation inhibits Pin1’s isomerization of ATR in the cytoplasm. Cis-ATR (ATR-H) then accumulates at the outer mitochondrial membrane, where it binds to and sequesters t-Bid. Because Bax and Bak cannot polymerize in the absence of tBid, cis-ATR suppresses cytochrome c release and apoptosis. In the nucleus, trans-ATR is the dominant isomer, interacting with ATRIP, RPA, and chromatin in the DNA damage repair (DDR) response. Protein phosphatases (PP2A) can dephosphorylate the Pin1 recognition motif and induce cis-ATR synthesis.
4. ATR Regulation of Nucleus Mechanics
Nuclear mechanosensing and mechanical features of the nucleus influence genome integrity, nuclear architecture, gene expression, cell migration, and differentiation [68,69]. ATR is a transcription factor that responds to mechanical stress at the nuclear envelope and mediates the envelope-associated repair of aberrant topological DNA states. Alteration of nuclear flexibility and YAP delocalization is observed in ATR-defective cells. ATR-defective nuclei collapse when subjected to mechanical stress or interstitial migration, accumulating nuclear envelope ruptures and perinuclear cGAS, indicating loss of nuclear envelope integrity and an abnormal perinuclear chromatin state. Furthermore, during development and in the metastatic spread of circulating tumor cells, ATR-defective cells have a defect in neuronal migration. Furthermore, the mechanical coupling of the cytoskeleton to the nuclear envelope and the accompanying regulation of the envelope–chromosome association is ensured by ATR [70].
5. ATR Regulation of Autophagy
DNA damage has been linked to autophagy via ATR/Chk1/RhoB-mediated lysosomal recruitment of the TSC complex and subsequent mTORC1 suppression. UV light (UV) or the alkylating chemical methyl methane sulphonate (MMS) damage DNA, causing Chk1 to phosphorylate the small GTPase RhoB. Phosphorylation of RhoB boosts its interaction with TSC2 and sumoylation by PIAS1, both of which are essential for the RhoB/TSC complex to translocate to lysosomes. mTORC1 is thereby blocked, and autophagy is induced. RhoB knockout drastically reduces TSC complex lysosomal translocation and DNA damage-induced autophagy, both of which are dependent on ATR–Chk1-mediated RhoB phosphorylation and sumoylation [71]. By acting on the transcriptional factor GATA4, ATR has also been associated with transcription regulation [72], which is susceptible to degradation by the autophagy-related factor p62 (also known as sequestosome 1 (SQSTM1)). In fibroblasts, ATR has been reported to have a negative effect on p62 [73]. In addition, a reduction in GATA4 levels and changes in gene expression associated with senescence and inflammation are observed when cells are treated with an ATR inhibitor [73].
6. The cis-ATR Anti-Apoptotic Role Drives Oncogenesis in Dividing Cells
Cancer is characterized by deregulated cell proliferation, which develops when there is an imbalance in the normal cell cycle regulation to govern the pace and integrity of cell division and growth. Furthermore, because cis-ATR has antiapoptotic properties, scholars surmise that it may have an oncogenic role, whereas Pin1 may have tumor-suppressive properties in relation to ATR’s anti-apoptotic activity at the mitochondria. If cis-ATR is the dominant cytoplasmic form, it may inhibit mitochondrial apoptosis, allowing injured cells to survive and mutate even when DNA damage repair is insufficient, and the aberrant cells are intended to die by apoptosis. This evasion of apoptosis is a key feature of cancer cells, allowing them to accumulate the mutations that define genomic instability and, eventually, carcinogenesis. However, if Pin1 activity is elevated and trans-ATR is the dominant form of ATR in the cytoplasm, programmed death will occur in cells that are too badly damaged for effective DNA repair before mutations can be transmitted. Thus, lowering cytosolic cis-ATR prevents the accumulation of cells with DNA damage, which could be passed on to daughter cells and induce carcinogenesis.
The current knowledge is based mostly on data showing that Pin1 is overexpressed/activated in most malignancies and cancer stem cells, with correspondingly poor prognoses [72,74,75,76,77,78,79,80]. Pin1 also promotes the expression of multiple oncogenes while suppressing the expression of several tumor suppressor genes [81]. Pin1 overexpression or activation can be blocked genetically or chemically with juglone [82], all-trans retinoic acid [34] (ATRA), or KPT-6566 [83], and Pin1 inhibitors have been shown to reduce malignancies when tested [84,85,86,87,88,89,90]. However, chemically suppressing Pin1 presents numerous complications, particularly with retinoids (e.g., ATRA), the most used clinical inhibitor. Low medication bioavailability, clinical relapse, and retinoid resistance are examples of these [91,92,93,94,95].
Following UV-induced DNA damage, the phosphatase PP2A dephosphorylates ATR at Ser428. Following UV irradiation, PP2A was shown to be involved in the decrease of pATR (S428) in the cytoplasm [64]. Because Pin1 function needs the Ser428–Pro429 recognition site in ATR to be phosphorylated, PP2A inhibits Pin1 activity by depleting cytoplasmic pATR at S428. Another layer of regulatory complexity to the DDR process is associated with this PP2A monitoring for ATR phosphorylation at the Pin1 motif recognition. In the event of DNA damage, PP2A interacts with ATR to dephosphorylate its Pin 1 recognition motif to prevent further isomerization of cis to trans ATR in the cytoplasm, resulting in accumulation of cis ATR-H in the cytoplasm. It was found that the accumulation of cis ATRH in the cytoplasm and mitochondria, following DNA damage, is associated with PP2A activity. Mitochondrial translocation ATR-H is an antiapoptotic protein that interacts with tBid to inhibit apoptosis activation and to reduce apoptotic cell death caused by DNA damage [64].
7. ATR as Therapeutic Target
7.1. Chemotherapeutic Effect by ATR–CHK1 Inhibition
The ability of ATR to limit oncogenesis in its early stages, by acting as a barrier to the proliferation of aberrant cells, occurs principally through p53 activation. The activation may lead to DNA repair and checkpoint arrest [102]. The fundamental theory is that ATR/CHK1 inhibitors, particularly in p53-deficient cells, increase tumor cell death by cytotoxic drugs or radiation by interrupting cell cycle checkpoints [103,104]. UCN-01 (7-hydroxystaurosporine) is the first CHK1 inhibitor with broad-spectrum activity against the protein kinase C family. In addition, UCN-01 lacks selectivity and has a long half-life, limiting its potential applications, since it binds to alpha acidic glycoprotein, which causes hyperglycemia, and has a long half-life [105,106]. XL844, an ATP-competitive inhibitor of CHK1, CHK2, VEGFR-2, and VEGFR-3, is quite effective. XL844 is designed to prevent CDC25A degradation, bypass S-Phase checkpoints, and increase DNA damage when used with gemcitabine. In the case of xenografts and in vitro, XL844 increases gemcitabine activity. The clinical study of XL844NCT00479175 and NCT00234481 was called off for reasons that are not yet known [107,108].
7.2. ATR Kinase Inhibitors
ATR belongs to the PI3K-related kinase (PIKK) family of enzymes, along with ATM, mTOR, DNA-PKcs, SMG1, and PI3K (Phosphatidyl Inositol 3 Kinase). Caffeine was the first ATR inhibitor to be identified that disrupted DNA damage induced cell cycle arrest and sensitized cells with DNA damage [117,128]. However, since the concentrations that prevent ATR and ATM are toxic, this medicine cannot be used in clinical trials, as it is also preventing ATM and PI3K family members.
The first potent and selective inhibitor, VE821, was reported by Vertex Pharmaceuticals in 2011. In comparison to ATM, PI3K, DNA-PK, and mTOR [129], VE-821 has >100-fold greater selectivity for ATR, and its analog VE-822 (VX-970) has been further enhanced with higher solubility, potency, selectivity, and pharmacodynamic characteristics. Preclinical studies showed that these agents potently sensitize several cancer cells lines to cisplatin, ionizing radiation, gemcitabine, PARP inhibitors, and topoisomerase I poisons etoposide and oxaliplatin in vitro [130,131,132,133,134,135].
Very selective, potent ATR inhibitors are also in development by AstraZeneca. In 2103, AZ20 was first reported [43]. The analog of AZ20, AZD6738, was described in 2013 as an orally accessible drug with improved solubility and pharmacodynamic qualities [136]. Compared to other types of PIKK, AZD6738’s selectivity is excellent for ATR [137].
7.3. ATR Kinase Inhibitors in Clinical Trials
In 2009, the first report on ATR-selective small-molecule inhibitors was published [118]. Schisandrin B, a naturally occurring dibenzo cyclooctadiene lignan found in the medicinal herb Schisandra chinensis, was found to be a selective inhibitor of ATR [118]. Schisandrin B inhibited UV-induced intra-S-phase and G2/M cell cycle checkpoints, and the cytotoxicity in human lung cancer cells was increased upon UV radiation. The inhibitory potency against ATR, on the other hand, was modest and required the use of large drug concentrations (30 M for cellular tests).
A more potent ATR inhibitor, NU6027, was reported in 2011 and was demonstrated to sensitize several breast and ovarian cancer cell lines to IR (insulin resistance) and several chemotherapeutic agents. However, this drug was originally created as a CDK2 inhibitor and is not ATR selective.
Toledo et al. also published the findings of a cell-based chemical library screening technique for the identification of effective ATR inhibitors in 2011 [34]. One of the compounds identified to possess significant inhibitory activity against ATR kinase was NVP-BEZ235, a drug originally introduced as a highly potent dual inhibitor of PI3K and MTOR with considerable in vivo anti-tumor activity [140]. NVP-BEZ235 has been demonstrated to be markedly radiosensitive to Ras-overexpressing tumors [141].
Vertex Pharmaceuticals discovered the first class of effective and selective ATR kinase inhibitors during a high-throughput screening strategy [133]. VE-821 was found to be a powerful inhibitor of ATP-like effects on ATR, but it had little to no interaction with other PIKKs such as ATM, DNA, and MTOR [44]. In the colorectal cancer cell line HCT116, VE-821 reduced phosphorylation of the ATR downstream target CHK1 at Ser345 and showed excellent constructive collaboration with genotoxic drugs from several classes. Chemosensitization was particularly evident with DNA cross-linking agents such as cisplatin, and it was further improved by p53 knockdown in ATM-deficient cells or in conjunction with the specific ATM inhibitor KU-55933.
AstraZeneca’s AZD6738 is a second ATR inhibitor now in clinical development. AZD6738 is an analogue of AZ20, a strong and selective ATR inhibitor that has been demonstrated to have significant single agent activity in vivo at well-tolerated doses in MRE 11A-deficient LVO xenografts [42,146]. AZD6738 possesses significantly improved solubility, bioavailability, and pharmacokinetic properties compared to AZ20 and is suitable for oral dosing [140]. In vitro, it suppresses the phosphorylation of the ATR downstream target CHK1 while boosting the phosphorylation of the DNA DSB marker γH2AX. The combination of this compound with carboplatin or IR has shown significantly increased antitumor growth inhibitory activity in in vivo studies. AZD6738 also showed single-agent anti-tumor efficacy in ATM-deficient but not ATM-proficient xenograft mice [136,147]. This anti-tumor effect was linked to a sustained rise in γH2AX staining in tumor tissue but only a transient increase in normal tissues such as bone marrow or the gut. This shows that a positive therapeutic index might be obtained, which is optimistic for the future clinical development of this drug.
8. ATR Isomerization Potential Therapeutic Target
The most commonly used ATR inhibitors in cancer clinical studies are specific inhibitors of the enzyme ATR kinase, which plays a key role in the DNA damage checkpoint function of ATR in the nucleus. These inhibitors have no effect on cis-ATR’s antiapoptotic activity, since the new inhibitor of cis-ATR at mitochondria, which is independent from ATR kinase activation [63], is not affected. Such a protein target that is novel and effective in the treatment of cancer could be cis-ATR (ATR-H), potentially. Cis-ATR is not, as a matter of principle, mutagenic, but it allows cancer cells to evade apoptotic activation, which is particularly important for carcinogenesis. Cancerous cells may be resistant to death because they contain proportionally more cytoplasmic cis-ATR than healthy cells, especially when exposed to chemo- or radiotherapy, or because they have less Pin1 or less Ser428 phosphorylation in ATR [148]. In support, reduced levels of pSer428 ATR in the cytoplasm of advanced epithelial ovarian cancer cells are correlated with poor prognosis [149]. As a result, using irradiation or chemotherapy to target cis-ATR as an adjuvant treatment for cancer should preferentially kill cis-ATR-dependent cancer cells while having little influence on the normal activities of nuclear trans-ATR in cells. To guarantee cellular survival and normality, ATR is a crucial protein [31] that consists of cis and trans isomers that are normally active but exist in a fragile balance.
9. Conclusions
The cellular functions of ATR are supported by two major activities: its kinase activity, and kinase-independent antiapoptotic activity directly at the mitochondria. ATR functions as a basal kinase and is an important part of repairing DNA, regulating the cell cycle, and apoptosis. It has a wide variety of therapeutic functions. In conclusion, ATR kinase has emerged as a promising target for cancer therapy, and the area of ATR inhibition (ATRi) is quickly expanding, with multiple early-phase clinical studies now underway.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241411684
This entry is offline, you can click here to edit this entry!
