1. Introduction
To date, noble-metal nanoparticles have been successfully incorporated with polymers [
150,
151,
152], oxides (e.g., SiO
2 [
153], Al
2O
3, TiO
2 and CeO
2 [
154] Nb
2O
5, Ta
2O
5, and ZrO
2 [
155]), metal–organic frameworks (e.g., ZIF-8, MIL-101-NH2) [
156,
157] and carbon materials (e.g., carbon black, carbon nanotubes, graphene) [
158,
159,
160,
161]. Over the past decades, carbon materials have been recognized as an ideal substrate because of their extraordinary physical and chemical properties and their universal availability, processibility, environmental friendliness, and relative stability in both acidic and basic media [
162]. At this point, it should be added that carbonaceous materials (CMs) have a large specific surface area, high porosity, excellent electron conductivity, relative chemical inertness, good thermal stability under an inert atmosphere, an intrinsic hydrophobic nature, and the presence of vast functional groups that facilitate metal loading [
159,
163].
Depending on distinct types of crystal structures, carbon atoms can form a variety of allotropes with different properties [
164]. In particular, carbon nanomaterials, such as 0D fullerenes, 1D carbon nanotubes, 2D graphene, etc., have dynamized research in the field of electrocatalysis. Built on them, NMNPs/CMs nanocomposites are an ideal option for various electrochemical reactions in energy conversion and storage, including hydrogen evolution and oxygen reduction. These materials withstand various types of electrochemical oxidation reactions, which reduce the lifetime of electrocatalysts due to sintering and poisoning effects [
165,
166].
In conventional systems, carbon black, which is a product produced by the pyrolysis of petroleum hydrocarbons, is usually used as a carrier for Pt nanoparticles. Due to its high availability and low cost, it is widely used in electrocatalysis applications [
167]. Common carbon blacks include acetylene black, Vulcan XC-72, Ketjen Black, etc. Their major physicochemical characteristics include specific surface area, electronic conductivity, large surface-to-volume ratio, stability, and surface functionality [
168,
169,
170,
171,
172].
2. Methods of Obtaining NMNPs/CMs Nanocomposites
Thus far, a lot of work has been devoted to developing new, efficient methods for the preparation of electrochemically active NMNPs/CMs nanocomposites. It was found that the smaller and more homogeneous the immobilized metal particles are, the better the electrocatalytic properties of the nanohybrid [
173,
174,
175]. Among the chemical-deposition methods of NMNPs on carbonaceous supports, one can distinguish: surface-functionalization methods, electrochemical deposition, and electroless deposition [
176,
177]. In this work, it has been decided that detailed descriptions of the syntheses of nanocomposites and their catalytic properties are to be presented for the most frequently described, i.e., widely understood, mesoporous carbon materials, carbon nanotubes, and graphene (
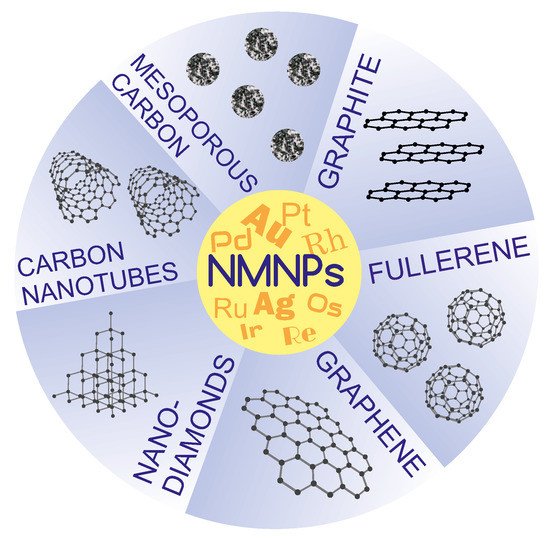
Figure 1). Examples of other carbon supports along with an indication of the synthesis method are summarized in
Table 1.
Figure 1. A schematic representation of various carbon supports for NMNPs.
Two types of procedures can be used depending on the requirement. In type 1: previously synthesized metal nanoparticles are deposited on carbon substrates. In type 2: the formation of nanoparticles and their deposition on carbon substrates occurs during a single process. These two processes can be based on the creation of covalent or noncovalent interactions between individual elements of nanocomponents. Both functionalization processes serve to enhance the number of active binding sites for the deposition of metal-nanoparticle catalysts and also to improve the dispersibility of the carbon substrate in water or solvents. These aspects ultimately enhance the catalytic effect [
217,
218]. In the case of noncovalent functionalization, the hybridization of the material in question remains unchanged. It also has little effect on its electronic properties, as a result of the weak van der Waals forces of attraction between the adsorbates and carbon substrates [
176,
177,
219]. In the case of covalent functionalization, different types of binding sites are generated. One form of covalent functionalization is oxidative means, during which oxygen groups such as carboxyl, carbonyl and hydroxyl groups, are formed [
177,
220]. They stabilize the dispersion of carbonaceous materials in polar solvents and provide active sites for metal adsorption. Such functionalized carbon materials with noble metal precursors can be further reduced by the addition of reducing agents (e.g., ethylene glycol (EG), NaBH
4, H
2, formic acid (HCOOH), etc.) to form NMNPs/CMs hybrids [
221,
222]. The synthesis process can be significantly accelerated by using an irradiation-assisted technique, for example, visible light or microwave irradiation [
223,
224]. Particularly noteworthy is the microwave-assisted polyol method, which has numerous advantages such as ease of procedure, speed and safety [
225,
226].
In the electrochemical method, the MNNPs/CMs nanocompounds are obtained via the reduction of noble metal complexes, such as H[AuCl
4], H
2[PtCl
4], or (NH
4)
2[PdCl
4], by electrons. It consists of the following steps: deposition of carbon material on the electrode, immersion of the carbon-coated electrode in an electrolytic solution containing metallic precursors, and application of an electrochemical potential. Carbonaceous materials act as molecular conductors to provide support for the deposited NMNPs. It should be noted that, in this case, CMs do not react with noble metal salts. The nucleation process and subsequent growth of NPs can be effectively controlled by adjusting electrodeposition parameters such as nucleation potential, deposition time, metal salt concentration, etc., [
176,
177,
204,
206,
227,
228].
The electrodeless deposition method constitutes a chemical process involving a direct redox reaction, by electron transfer between metal ions (higher reducing potential), and a carbon support [
229,
230]. Due to the fact that such a process does not require any external reducing agents, electrodeless deposition is considered as a green strategy for producing metal NPs on carbon substrates [
231].
2.1. Noble-Metal Nanoparticles on Mesoporous Carbon Materials
Carbon materials with mesoporous characteristics (2 nm < pore sizes < 50 nm) have received considerable attention in electrocatalytic applications. They can facilitate the transport of reactants to the electrocatalysts and simultaneously exhibit large surface areas and low charge-transfer resistance [
232,
233,
234]. On this basis, numerous examples of the use of mesoporous carbon materials [
186,
235,
236] have attracted much interest as electrocatalyst supports for fuel-cell applications. Using ordered and highly ordered mesopoporous carbon materials as substrates for Pt and PtRu nanoparticles, respectively, it was shown that the size and distribution of mesopores play an important role in electrochemical reactions [
236,
237]. Qi et al. synthesized PtRu nanoparticles with graphitic mesoporous carbon (GMC) substrates using a chemical reduction process, with H
2PtCl
6 and RuCl
3 as precursor nanoparticles [
232]. The study involved the use of GMCs with different pore sizes, and the results clearly indicated that this parameter affects the performance of direct methanol fuel cells. Carbon aerogels (CA), which can be synthesized from cellulosic biomass, are another favorable porous material for fuel-cell-energy-storage and conversion applications [
238,
239,
240,
241]. An example is the work of Gu et al., in which the effect of CA pore size on the deposition of Pt NPs was reported for proton-exchange-membrane-fuel-cell (PEMFC) applications [
198]. In the initial stage, CA was impregnated with Vulcan XC-72R carbon, and then platinum nanoparticles (from H
2PtCl
6) were attached to the prepared system by chemical reduction with NaBH
4. In turn, Cheng et al. manipulated the potential (potentiostatic deposition or square wave potential deposition) produced Au nanostructures with different sizes and shapes on the surface of carbon fiber paper electrodes [
242]. Pt monolayers were deposited onto the resulting urchin-like nanostructures using a surface-limited redox replacement method. The resulting systems were tested in a methanol electrooxidation reaction.
An important step during the synthesis of nanocomposites is the regulation of the surface properties of the MC through different surface-modification methods before the deposition of metal NPs [
186,
243]. For example, Su et al. deposited uniformly dispersed Pt NPs on the surface of N-doped porous carbon nanospheres (PCNs) [
244]. Pt/PCNs hybrids were synthesized by the reduction method using EG as a reducing agent and H
2PtCl
4 as precursor. The process was assisted by microwave irradiation for 3 min. The authors found that Pt/PCNs exhibited increased activity in the methanol oxidation reaction (MOR) than the commercial E-TEK catalyst. In turn, in Ott’s group, N-functionalized Ketjen Black carbon powder was used as a substrate for Pt nanoparticles [
180]. Modification of the carbon support was carried out by pre-oxidizing the pristine carbon in concentrated HNO
3 at 70, 200, 400 and 600
∘C. That modification contributed to the formation of carboxylic, hydroxylic and NO
x groups at the surface and also altered the meso- and microporous structure of the carbon supports. An increase in nitrogen content and a higher proportion of mesopores for media subjected to higher temperatures were observed. Following this, the polyol method was used, in which the carbon and Pt precursor (H
2PtCl
6) were dispersed in ethylene glycol and reduced at 120
∘C for 2 h. As a result, Pt NPs were deposited on the outer and inner surface of carbon powder particles, and, thus, a high power density in the fuel-cell catalyst, with high stability under voltage cycling, was obtained.
A parameter that also improves fuel-cell performance is the method of catalyst synthesis [
245]. Harzer et al. determined the performance of PEMFC cells depending on the way platinum nanoparticles were distributed on Ketjen Black. Using a polyol method and reducing a highly dilute platinum precursor in ethylene glycol, Pt nanoparticles were obtained on the outer carbon surface and in solution. In contrast, using a prewetting method in which the carbon support is impregnated with a highly concentrated Pt precursor solution, nanoparticles were obtained inside the pores of the carbon particles. The catalyst with more Pt particles deposited on the outer surface of the carbon achieved better results.
2.2. Noble-Metal Nanoparticles on Carbon Nanotubes
Discovered by Iijimain in 1991, carbon nanotubes (CNTs) are one of the most widely used substrates for the formation of NMNPs/CMs complexes. They are defined as the ordered, hollow graphene-based nanomaterials made up of carbon sp
2 - hybridised atoms. They can be classified into the following two categories: (1) single-walled carbon nanotubes (SWCNTs), consisting of a single sheet of carbon that has been rotated into a tubular form, and (2) multi-walled carbon nanotubes (MWCNTs), which are comprised of several concentric SWCNTs having a mutual longitudinal axis [
159,
246]. The diameter of CNTs is in the nanometer scale, while their length can reach several microns. Numerous examples of NMNPs/CNTs nanohybrids have been reported in the literature for catalytic applications. The first application of NMNPs/CNTs nanocomposites in heterogeneous catalysis dates back to 1994 [
247]. This kind of composite material advantageously integrates the unique properties of individual materials and exhibits some innovative features resulting from the interactions between CNTs and NMNPs. These features directly translate into numerous attractive applications in many fields, especially in catalysis, fuel cells, and environmental-contaminant sensing [
159,
248,
249,
250,
251].
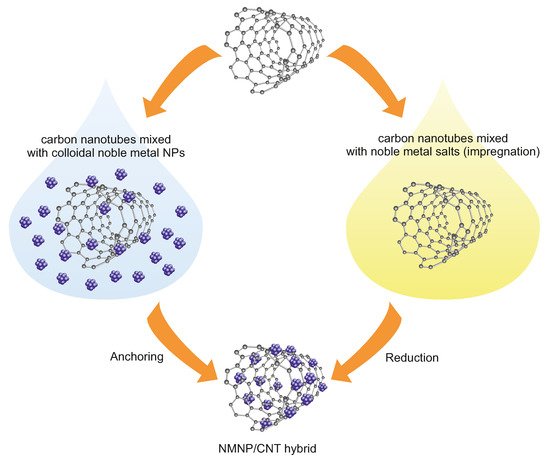
Obtaining small size, dispersed particles of noble-metal NPs on CNTs is desirable due to high catalytic activity and also for economic reasons. Despite the fact that CNTs exhibit excellent electrical, mechanical, and thermal properties, however, they are chemically inactive and hydrophobic. As a consequence of this, they often do not have enough binding sites for anchoring guest molecules, which results in low dispersion and a large particle size of nanoparticles. Therefore, functionalization of the external surfaces of CNTs is generally carried out [
252]. Preformed NMNPs can be deposited on functionalized CNTs or functionalization and synthesis can be carried out in a single process of the formation of NMNPs/CNTs complexes (
Figure 2). The surface modification of carbon nanotubes can be performed either covalently or noncovalently [
253]. Such CNTs functionalization methods serve both to increase the number of active binding sites for NMNP deposition and to improve the dispersibility of CNTs in solvents, which enhances the catalytic effects [
217,
218].
Figure 2. Scheme of the deposition of NMNPs on carbon nanotubes.
Covalent functionalization of CNTs is often carried out via an aggressive oxidation treatment with a HNO
3 or HNO
3/H
2SO
4 mixture [
253]. This process contributes to the formation of several functional groups, such as carboxylic, carbonyl, and hydroxyl groups, on the surface of nanotubes. It can also be carried out by pretreatment of CNTs in HCl, HF, KMnO
4 or H
2O
2 [
254]. Then, covalently functionalized CNTs often undergo subsequent functionalization processes to control the size and dispersion of the NMNPs deposited on them. That approach was used by Wang’s group [
255]. In the first step, they functionalized the nanotubes with COOH groups. They then attached amine-terminated ionic liquids (NH
2-IL) to the functionalized nanotubes. The gold salt [AuCl
4]
− was adsorbed to the thus-formed amide bond between MWCNTs-COOH and NH
2-IL through electrostatic interaction and ion exchange. As a result of this process, well dispersed 1–2 nm Au NPs were obtained.
Noncovalent functionalization involves the attraction of the hydrophobic end of an adsorbed molecule to the walls of CNTs via van der Waals forces or π–π interactions [
176]. This is carried out without disturbing the electron structure of the CNTs, as the covalent bonds are not affected. In this case, the following may be used aromatic organic compounds such as derivatives of pyrene, thionine, or triphenylphosphine, and they contribute to the formation of several functional groups such as thiol, amine, or carboxyl groups, which can be used as the linkers to anchor NMNPs onto CNTs surfaces. In paper [
256], it was shown that, by using the in situ polymerization method, it was possible to obtain a homogeneous polymer coating on the surface of MWCNTs, which allows for better dispersion of the nanotubes. An example of such noncovalent functionalization is the modification of carbon nanotubes presented by Zheng [
257]. The synthesis of Pd/MWCNTs nanocomposites with particle sizes of 3 nm was achieved by π–π stacking interactions of MWCNTs and naphthalen-1-ylmethylphosphonic acid (NYPA).On such functionalized carbon nanotubes, Pd nanoparticles were deposited by means of a homogeneous precipitation–reduction reaction method by using PdCl
2 as a noble metal precursor and NaBH
4 as a reduction agent.
Using the same method, it is also possible to form bimetallic-based composites. For example, by reduction with H
2PtCl
6, the addition of ruthenium and molybdenum precursors (Ru
3(CO)
12, Mo(CO)
6), followed by annealing for 2 h at 400
∘C in a N
2 atmosphere, bilemetallic Pt-Ru/SWCNTs and Pt-Mo/SWCNTs composites were obtained. Moreover, the as-synthesized Pt-Ru/SWCNTs composite showed better current and power densities than Pt/SWCNTs catalysts [
258]. Another bimetallic electrocatalyst that plays an important role in the development of direct-methanol-fuel-cell applications is the PtIr/MWCNTs composite [
259].
Another way to improve physical and catalytic properties is doping, realized by replacing the carbon atoms in carbon nanotubes with other elements such as nitrogen, phosphorus or boron. For example, Yu et al. [
260] reported doping of MWCNTs with phosphorus (P) and nitrogen (N), which improves their durability and increases their electrocatalytic activity. In turn, Jin et al. reported that Pt/CNTs doped with selenium atoms show long-term stability and good activity in comparison with a commercial Pt/C catalyst [
261]. Nitrogen can be used both as a dopant and a surfactant in the growth of CNTs [
262]. Nitrogen-functionalized CNTs (N-CNTs) have a high number of surface nucleation sites, which allow the anchorage and high dispersion of the noble metal particles [
263]. N-CNTs, as substrate material, possess high resistance to surface oxide corrosion, which is an attractive feature, e.g., in oxygen reduction reactions (ORR) [
184]. Noteworthy is also paper [
264], which shows that boron doping increases the binding energies of transition metals to CNTs supports more than nitrogen. In turn, Rajala and co-workers fabricated platinum nanowires on SWCNTs (Pt NWs/SWCNTs) then pretreated with ozone, which resulted in the formation of polar surface groups on the carbon nanotubes. The fabricated Pt NWs/SWCNTs-O
3 composites were more hydrophilic in nature and outperformed in the hydrogen evolution reaction (HER) than non-ozonized compounds [
265].
The current trend is the replacement of noble metal nanoparticles in NMNPs/CNTs complexes with cost-effective alternatives, including, for example, nickel. Nickel-doped materials have relatively high electrochemical activity (e.g., ORR, CO
2 reduction) and are low-cost [
184]. Zhang et al. developed Pd nanoparticles assembled on Ni- and N-doped carbon nanotubes. The resulting CNTs-based composite with homogeneous and monodispersed Pd and Ni particles (2–5 nm and <1 nm, respectively) achieved much better hydrogen evolution reaction (HER) activity compared with the commercial Pd/C sample.
By using an electrochemical method, a very high purity of nanoparticles and their good adhesion to CNTs substrate can be ensured. However, the NMNPs/CNTs nanocomposites prepared by this method usually receive particles with big particles size (between 10 and 100 nm), as shown in He’s work [
228]. Pt or bimetallic Pt–Ru nanoparticles were electrodeposited on the CNTs by the potentiostatic method from H
2SO
4 aqueous solution with ruthenium chloride and chloroplatinic acid. The nanoparticles obtained by this method, although characterized by high purity, also had a grain diameter larger than 60 nm. In order to decrease the size of the metal NPs on the CNTs, Tsai et al. [
266] synthesized a Pt and a Pt–Ru/CNTs by potentiostatic electrodeposition in mixed sulfuric acid and ethylene glycol containing aqueous electrolytes. It was found that the addition of EG led to the formation of uniformly dispersed and non-agglomerating Pt and Ru NPs with small sizes, ranging from approximately 4.5–9.5 nm and 4.8–5.2 nm for Pt and Pt–Ru, respectively. Grain size reduction can also be achieved by using methods such as cyclic potential scanning [
267], pulsed electrodeposition [
268], ultrasonic-electrodeposition [
269] and a co-electrodeposition/stripping protocol [
270].
The formation of NMNPs/CNTs complexes is also possible by using electroless deposition. An example is the use of the one-pot method, which uses a redox reaction between metal ions and reduced CNTs [
271]. Using this procedure, Au and Pd NPs were successfully anchored to the surface of MWCNTs and SWCNTs. Another method that overcomes the limitations of classical electroless deposition is substrate-enhanced electroless deposition (SEED). In the SEED method, CNTs are supported with metal substrates whose redox potential is suitably lower than that of the metal species being reduced. In this case, CNTs are no longer a reducing agent and act only as cathodes and templates for metal deposition from the corresponding noble-metal salts [
214].
2.3. Noble-Metal Nanoparticles on Graphene
In addition to carbon nanotubes, graphene (GR) as carbon support also improves the catalytic activity and stability of supported Pt catalysts compared to mesoporous counterparts through enhanced electrical conductivity and metal–substrate interaction [
181]. Graphene is a single-atom-thick two-dimensional carbon nanosheet of sp
2 bonded carbon atoms packed into a honeycomb lattice that was first synthesized in 2004 by Geim and Novoselov [
272]. In 2010, this discovery was awarded the Nobel Prize in Physics for its significant contributions to the development of graphene-based catalysts [
273]. Over the years, graphene, as well as its derivatives, including graphene oxide (GO) and reduced graphene oxide (rGO), have become promising candidates for many applications such as batteries, photovoltaic devices, biosensors, supercapacitors and fuel cells. This wide range of applicability is influenced by its unique properties such as large surface area, thermal conductivity, high electron mobility and good stability [
159,
177,
274,
275]. Significantly, the numerous oxygen-containing groups present in GO and rGO provide many opportunities for further functionalization and modification [
276,
277,
278,
279]. Graphene is an excellent building block in the fabrication of various nanocomposites representing the most recent advance in many fields of chemistry, physics, and electronics [
219,
274,
275,
280,
281]. Combining GR with noble-metal nanoparticles improves electrochemical performance and provides perfect thermal stability, which is important in electrocatalytic applications (e.g., oxygen reduction and hydrogen/oxygen evolution reactions) [
282,
283].
The preparation of GR-based noble-metal nanocomposites by using wet-chemical synthesis methods has many advantages, such as an economic cost of production, high yield, mass production and commonness. The most popular strategy is the direct chemical reduction of a noble metal precursor (e.g., HAuCl
4, AgNO
3, H
2PdCl
4 or K
2PtCl
4) in the presence of graphene and its derivative sheets using a reducing agent such as amines, NaBH
4, and ascorbic acid [
284,
285,
286]. For example, Iqbal’s group prepared 34 nm mesoporous Pd nanoparticles on rGO sheets modified by the block copolymer F127 [
287]. This block copolymer has served as a template for better dispersion of Pd nanoparticles. As a precursor to palladium particles, H
2PdCl
4 was used, which was reduced by ascorbic acid. By using direct chemical reduction, it is also possible to synthesize GR-based multimetallic noble-metal nanocomposites. For example, Pt-Pd supported on rGO were obtained by the reduction of a noble-metal precursor (H
2PtCl
6 and PdCl
2) by ascorbic acid and octylphenoxypolyethoxyethanol (NP-40) as a soft template [
288]. Vilian et al. reported the synthesis of Pt-Au/rGO nanohybrids by a direct chemical-reduction methodology. The reported methanol oxidation is found to exhibit excellent electrocatalytic performance, reliability, and stability, surpassing that of several reported modified electrodes that can also be used for platinum-based catalysts in fuel-cell applications [
289].
The chemical reduction process can also be assisted by microwave irradiation for the synthesis of chemically converted graphene sheets and metal nanoparticles dispersed on them [
290]. In turn, the sonochemical method has been used, for example, in the development of Au/GR nanocomposite [
291]. In this study, by using an ultrasonication probe of 20 kHz on the surface of exfoliated few-layer graphene sheets, the in situ growth of gold nanoparticles (Au NPs) after the reduction of gold chloride took place. Alternatively, Huang et al. synthesized Pd/rGO nanohybrids with ~3 nm nanoparticles using a one-pot photoassisted citrate reduction. In the process shown, the mixture of GO solution, Na
2PdCl
4, sodium citrate, and deionized water was irradiated by a 500 W high-pressure mercury lamp for 12 h. This synthetic approach allows for the formation onto the rGO surface of Pd nanoparticles with the desired size with excellent activity and stability of complexes in oxygen reduction and ethanol oxidation reactions. The prepared Pd/rGO nanocomposite exhibited 5.2 times higher mass activity for ethanol oxidation reaction than the commercial Pt/C catalyst [
292].
By analogy with other carbonaceous supports, it is possible to use an electroless method to prepare NMNPs/GR composites. In this case, graphene derivatives (GO or rGO) themselves can donate electrons to reduce the noble-metal precursors in an aqueous phase without any additional reductant. In accordance with this, metal precursors (e.g., HAuCl
4, H
2PdCl
4, or AgNO
3) can be reduced to form metallic Au, Pd, and Ag nanoparticles, respectively, solely by GO or rGO [
284,
293,
294]. An example would be the work of [
295], in the synthesis of the redox reaction between Au, Ag or Pd precursors and the partially reduced graphene oxide (prGO) in an aqueous solution. The as-obtained Au, Ag and Pd/prGO nanocomposites display excellent catalytic activities, and the size distributions of the Au, Ag, and Pd particles were 1–20 nm, 3–10 nm and 0.5–3 nm, respectively.
Interesting conclusions presented by Qin et al. claim that a heating treatment and strong alkaline conditions enhance the reducing ability of the hydroxyl groups on GO. In their work, Au/rGO nanocomposites were prepared through a one-pot strategy, conducted by heating a mixture of HAuCl
4, NaOH and graphene oxide solution at 90
∘C [
294]. It is also possible to obtain NMNPs/GR nanocomposites by using pure graphene without any additional groups. This strategy was proposed in a paper by Jeong et al., in which graphene was covered onto a reducing substrate (e.g., Si or Al) [
286]. As a result, Au/GR or Pt/GR nanocomposites could be prepared because electrons were transferred from the substrate, via graphene, to the precursors (HAuCl
4 or KPtCl
4). In Zou et al.’s paper, using a two-step electrochemical deposition method, spherical Au nanoparticles and a 3D flower-like structure graphene were obtained on a glassy carbon electrode (Au/rGE/GCE) [
296]. Another example was presented by Liu et al.: an electrochemically seed-mediated method by which sub-10 nm tetrahexahedral (THH) Pt NCs supported on graphene were synthesized. The obtained nanohybrids exhibited a higher mass activity than a commercial Pt/C catalyst for ethanol electrooxidation [
297].
All of the GR-based nanohybrid examples presented above focus on graphene and its derivatives’ 2D morphology. However, the practical application of graphene is associated with difficulties in the form of the stacking and folding of its sheets. In particular, it is hindered by 2D GR wrinkles that wrap around MNNPs. This limits electron and mass transport and makes its application in electrocatalysis much more difficult [
298]. Therefore, the focus has been on designing various 3D GR nanostructures (e.g., framework, network, foam, etc.). These treatments aim to minimize wrinkles as well as reduce agglomeration of nanoparticles [
299,
300,
301,
302]. Qiu et al., by using a layer-by-layer assembly method, presented a versatile synthesis strategy based on sacrificial templates to obtain three-dimensional graphene-assisted PtM (M = Fe, Co, Ni) nanospheres [
303]. In the first step, electrostatic attraction was used to wind 2D GO sheets on positively charged SiO2 nanospheres. Next, PtM (M = Fe, Co, Ni) alloy nanoparticles were deposited on the surface of 3D rGO nanospheres. Finally, after etching SiO
2, the 3D rGO-supported PtM hollow nanospheres were formed. These nanocomposites exhibit enhanced electrocatalytic activity, durability, and stability for methanol oxidation reactions (MOR), compared with commercial Pt/C. On the other hand, Yao et al. showed that Pd nanoparticles encapsulated in hollow microspheres of N-doped graphene, exhibited higher EOR activity in an alkaline medium than Pd/rGO [
304]. Additionally, it should be noted that the structure of N-doped GR hollow microspheres greatly facilitates the diffusion of reactants, which, in turn, improves the catalytic reactions [
305].
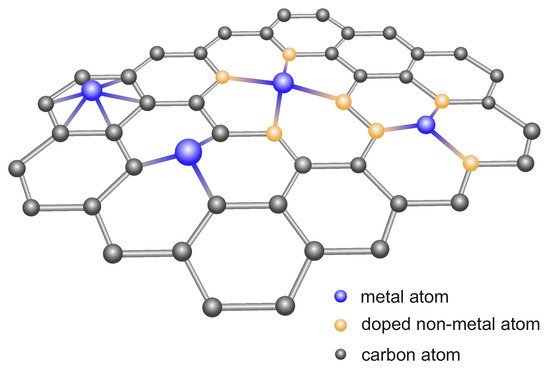
Reducing the particle size of noble metals positively affects the activity of the catalysts constructed on their basis, by significantly increasing the specific activity per metal atom. Therefore, single-atom catalysts (SACs) containing single-metal atoms anchored on supports are sought. The surface of pristine graphene and metal atoms are not firmly fixed, and they easily diffuse together to form nanoparticles [
306]. Therefore, obtaining dispersed metal atoms on pure GR is difficult. Accordingly, the surfaces of an ultrathin thickness and large specific surface area of 2D GR nanosheets have been doped with heteroatoms such as N, O, or S, which provide anchors for SAC (
Figure 3) [
307].
Figure 3. Scheme of doped graphene nanosheets with single-atom catalysts.
The atomic layer deposition (ALD) technique has become a promising method to obtain monoatomic catalysts on graphene derivatives [
306,
308,
309]. This cyclic process was based on sequential self-terminating reactions between a solid surface and gas-phase precursor molecules [
310]. For example, Sun et al. described the practical construction of isolated Pt atoms anchored in graphene nanosheets (GNSs) using the ALD method [
311]. In the construction process, oxygen and (methylcyclopentadienyl)-trimethyl platinum (MeCpPtMe
3) were used as precursors. In the first step, the number of oxygen-functional groups on the rGO surface was selected to form a thin Pt monolayer. Subsequently, oxygen exposure formed new surface oxygen on the pre-existing Pt layer. This process completed one cycle of a complete reaction. The morphology, size and loading density of platinum over graphene were controlled by simply tuning the cycles of the ALD (i.e., 50, 100, and 150 cycles). Finally, the best results, i.e., single Pt atoms, were obtained after 50 ALD cycles. All the discussed ALDPt/GNSs catalysts show several-times-higher activity for MOR than the Pt/C catalyst. Among them, ALD50Pt/GNS is more than 9.5 times more active than the Pt/C catalyst. Another example of using the ALD method was presented by Yan et al. They described the growth of single Pd atoms anchored to phenyl groups on rGO substrates [
309]. Using the same method, they also presented the deposition of Pt
2 dimers on graphene [
312].
Wet impregnation is another method for the synthesis of single noble-metal atoms supported on graphene [
313,
314,
315]. For example, Zhang et al. synthesized single Ru atoms on N-doped GD [
314]. For example, Zhang et al. synthesized single Ru atoms on N-doped GO by using the Ru(NH
3)
6Cl
3 as a precursor. The mixture thus formed was lyophilized to prevent the restacking of GO sheets. The resulting GO impregnated with Ru atoms was annealed in NH
3 gas at 750
∘C. This process led to the reduction of GO to form GR. Finally, nitrogen atoms were doped into the graphene plane as anchor sites for ruthenium atoms.
This entry is adapted from the peer-reviewed paper 10.3390/cryst12050584