Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Energy & Fuels
Hydrogen is emerging as a new energy vector outside of its traditional role and gaining more recognition internationally as a viable fuel route. Among the most potential renewable energy sources for hydrogen production are solar and wind. The production of H2 from renewable sources derived from agricultural or other waste streams increases the flexibility and improves the economics of distributed and semi-centralized reforming with little or no net greenhouse gas emissions.
- hydrogen
- blue hydrogen
- green hydrogen
1. Introduction
The tremendous worldwide economic and demographic growth that is taking place is behind the rise in energy demand. Power generation plays a significant role in each country’s industrial development. Fossil fuels provide for a sizable portion of the expanding energy demand; nevertheless, these conventional sources are in a very difficult situation because of their quick depletion. Increased global warming and CO2 emissions are the main side effects of exploiting these traditional fossil fuel resources [1]. Renewable energy sources are the most likely candidate to replace these conventional fuels because of the escalating environmental problems. Due to increased greenhouse gas (GHG) emissions, environmental concerns and global warming, the globe must transition from conventional to renewable energy sources.
Renewable energy sources including solar, wind, hydro, geothermal, ocean thermal energy conversion (OTEC) and biomass are the leading candidates to replace fossil fuels [2]. Hydrogen can make the most of these resources and be utilised not just as fuel but also as an energy transporter and a storage medium because certain attractive renewable energy sources, such as solar and wind, are sporadic. In order to attain net-zero CO2 emissions by 2050, it could also be crucial to decarbonize the main industries [3]. Hydrogen is becoming more known on a worldwide basis as a distinct energy source and possible fuel due to its carbon-free solutions.
The infrastructure for fuel storage and transportation that is now used for other chemical fuels is also considered as a potential option for hydrogen storage and delivery.
Clean hydrogen may be produced using a variety of home energy sources, including nuclear energy [4], natural gas [5], coal gasification [6], and renewable energy sources including solar [7], wind [8], biomass [9], geothermal [10], hydro [11] and OTEC [12].
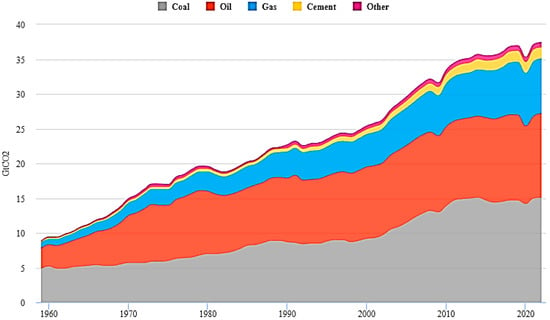
Figure 1 depicts the proportion of the world’s energy supply. A massive 35% of the world’s energy supply comes from coal, followed by a 23% share from gas, 16% from hydropower and 10% from nuclear. Renewable energy sources contribute around 13%, which includes 7% from wind energy, 4% from solar, around 2% from Hydrogen and the rest from biomass and biogas. Annual CO2 emissions from different types of fuels are shown in Figure 2.
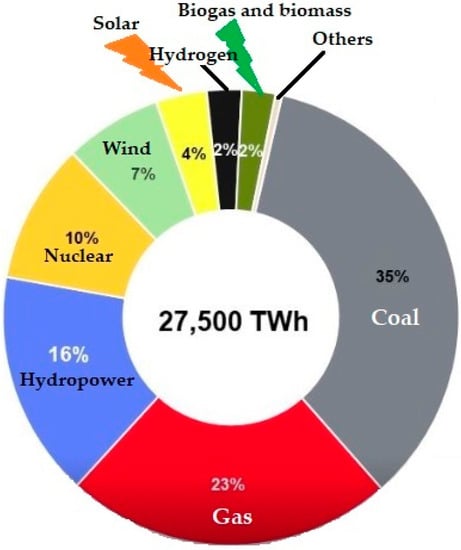
Figure 1. Global share of energy sources 2021 [13].
Coal, oil and gas combustion are the main sources of global emissions from fossil fuels. In 2022, coal will account for 40% of all global emissions from fossil fuels, more than any other fossil fuel. Gas production comes in third with a contribution of 21%, followed by cement production with a contribution of 4% and oil with a contribution of 32% of fossil CO2. Presently the contribution of Hydrogen in the energy sector is negligible and when hydrogen is produced from renewable energy sources the net CO2 emission is much lesser compared to other fuels. These percentages account for both the global use of each fossil fuel as well as variations in CO2 intensity. Oil and gas are the next highest emitters of CO2 per unit of heat or energy generated after coal. As depicted in Figure 1 and Figure 2, oil, coal and natural gas are traditional energy sources that dominate the world’s energy supply, power production and CO2 emissions.
A modern and almost established energy source is Hydrogen [17,18,19]. Table 1 lists comparisons of the main technical parameters of hydrogen with other fuels.
| Parameter | Hydrogen | Diesel | Methane |
|---|---|---|---|
| Density at STP (kg/m3) | 0.089 | 830.0 | 0.720 |
| Volumetric energy at STP (MJ/m3) | 1.07 × 10 | 3.5 × 104 | 3.3 × 10 |
| Net Lower Heating value (MJ/kg) | 119.9 | 42.5 | 45.8 |
| Boiling point (K) | 20.0 | 453–633 | 111.0 |
| Auto-ignition temperature (K) | 853 | ~523 | 813 |
| Minimum ignition energy in air at 1 bar & stoichiometry (mJ) | 0.020 | 0.240 | 0.290 |
| Stoichiometry air/fuel mass ratio | 34.4 | 14.5 | 17.2 |
| Quenching distance at NTP (mm) | 0.64 | - | 2.1 |
| Laminar flame speed in air at NTP (m/s) | 1.85 | 0.37–0.43 | 0.38 |
| Diffusion coefficient in air at STP (m2/s) | 85 × 10−7 | - | 19 × 10−7 |
| Flammability limits in air (% vol) | 4–76 | 0.6–5.5 | 5.3–15 |
| Adiabatic flame temperature at NTP (K) | 2480 | ~2300 | 2214 |
Among all other fuels, whether they be liquids or gases, hydrogen delivers the fastest burning rate. The hydrogen fuel cells provide a high-performance indication in terms of efficiency since they are not limited by the thermal Carnot cycle’s thermal efficiency restrictions. In order to support hydrogen-powered fuel cell and fuel cell-based hybrid automobiles, it is anticipated that a significant quantity of H2 refuelling stations will be built in 2022. Some of the key benefits of hydrogen include: effective conversion of energy; production through water splitting with no carbon emissions; synthesis of various intermediate fuels, including synthetic fuels and ammonia, followed by various chemical reactions; storage availability; ability to use in existing infrastructure for long-distance transportation; and a high LHV in comparison to other fuels.
Around the world, funding is already being considered for several hydrogen-based initiatives. To study offshore hydrogen generation, for instance, the OYSTER group was given 5 million euros [24]. Air Liquide [25] just put into operation the biggest green hydrogen generation facility in the world located in Quebec, Canada and generating up to 8.2 tonnes of green hydrogen a day (or around 3000 tonnes/annum). Although several green-hydrogen entrepreneurs have gigawatt-scale goals, the sector is still in its infancy. As a result, both the public and commercial sectors are making investments in the developing offshore wind hydrogen/ammonia industry.
Germany is planning a ground-breaking tender [26] for an offshore wind-hydrogen pilot in 2022 in order to achieve this. For experimental projects in the nation’s exclusive economic zone in the North Sea, Berlin has contributed 58 M. 5 million euros, given to the EU-based OYSTER collaboration to study offshore hydrogen production [27]. To perform a feasibility study on the ship-to-ship bunkering of green ammonia at the Port of Singapore, Maersk has inked a contract with a number of other foreign businesses [28]. The Nordic Green Ammonia Powered Ships (NoGAPS) project [29], worth NOK 8 million, was developed by the Nordic maritime sector with the goal of decarbonizing the transport of people and goods at Nordic ports and between sea and land. This was carried out in order to bring shipping in line with the Paris Climate Agreement. To make green hydrogen commercially viable, the government has unveiled 38 policies [30].
2. Hydrogen Production
Hydrogen production may be categorised into three main groups: green (based on renewable energy), blue (based on coal gasification and natural gas, along with CCS hydrogen generating systems) and red (based on fossil fuels alone) (based on conventional fossil fuels). Green hydrogen may also be created utilising renewable energy sources, despite the fact that the majority of it is currently produced via the CO2-intensive steam methane reforming process. Electrolysis is a typical method that uses an electrical current to separate water into oxygen and hydrogen and creates green hydrogen without any direct emissions of carbon dioxide. Renewable energy sources may be used to produce the necessary electricity. The expense of producing hydrogen, particularly for green hydrogen, is a significant hurdle. The cost of manufacturing hydrogen using steam reforming is around three times greater than the cost of producing one unit of energy using natural gas. Hydrogen will cost almost twice as much to produce using electrolysis with 5 cents/kWh of energy compared to hydrogen produced using natural gas. Lower hydrogen concentrations may be transported via the existing natural gas pipeline infrastructure, which will also assist in reducing CO2 emissions from the existing natural gas reforming plants.
There are a number of factors drawing attention to hydrogen fuel, but the following are the most important ones:
-
Different energy sources can be used to produce hydrogen.
-
Since hydrogen is the least polluting, using hydrogen in fuel cells or combustion processes results in the production of water.
-
All energy needs may be met by hydrogen, which is also utilised in residential applications, hydrogen fuel cell automobiles, energy carriers, and integrated heating and power generation systems. Hydrogen also covers all requirements for energy
By using off-grid offshore wind energy to generate clean fuel (hydrogen/ammonia), hydrogen may significantly contribute to the decarbonization of the marine sector. Energy may be transported and potentially stored by hydrogen, which is the best substance for both. Some important hydrogen generation techniques include: landfill gas dry reformation; coal gasification; H2S methane reformation; naphtha reformation; methane/natural gas pyrolysis; steam-iron process; steam reforming of waste oil; partial oxidation of heavy oil and coal; grid electrolysis of water; high-temperature water electrolysis; chloralkali electrolysis; solar and PV water electrolysis; photolysis of water; and biomass gasification.
According to the literature and study reports [34,35], there are three main forms of hydrogen generation based on the use of various technologies and proposed sources. The idea of colour has been developed as a result of the use of important sources in the generation of hydrogen. The production of hydrogen from fossil fuels results in the release of CO2 and other greenhouse gases. Grey hydrogen is the term used to describe this method of producing hydrogen and its used source [36]. Blue hydrogen came into being as a result of the use of grey hydrogen with carbon capture technology to lower the quantity of greenhouse gas emissions [37,38]. In order to produce hydrogen for sustainable transportation, fossil fuels including industrial gas, by-product gas and natural gas typically release pollutants and greenhouse gases into the atmosphere [39,40,41]. A variety of technical advancements for hydrogen generation have been documented in the literature as a means of reducing the pollutants released into the environment. Therefore, recent literature [42,43,44] has reported on the use of renewable energy resources for hydrogen production. Green hydrogen is another by-product of electrolysers made from renewable energy sources. It has also been noted that bioenergy sources such as the burning of biomass and biomethane can also create green hydrogen. Researchers and businesses are paying increasing attention to the improvement of green hydrogen generation, since green hydrogen produced using diverse approaches has net zero gas emissions [45,46].
2.1. Blue Hydrogen Production Methods
Current conventional hydrogen production facilities integrate carbon capture and storage (CCS) or carbon capture and utilisation (CCU) systems to collect the discharged CO2 emissions. Alternately, to fulfil the worldwide need for hydrogen, which is currently being satisfied by renewable energy sources, conventional hydrogen production pathways are employed. These approaches provide a sizable part of hydrogen. The majority of conventional hydrogen is produced from fossil fuels, mainly by partially oxidizing methane and reforming natural gas. Unprocessed natural gas contains gaseous hydrogen sulphide (H2S), which is removed from the gas during the first stage of processing by desulfurization. The Claus procedure is used to extract sulphur from gaseous H2S. In Claus plants, the gaseous hydrogen sulphide generated interacts with oxygen gas to extract sulphur. Another common technique for producing hydrogen is coal gasification. Air separation is the initial phase, which extracts oxygen from the air and delivers the same to the gasifier. One of the three frequently used methods for air separation: membrane separation, cryogenic air separation, or pressure swing adsorption, is employed. Coal pyrolysis follows the air separation process. In order to gasify coal, the pyrolysis stage needs two inputs: steam and oxygen. Coal is broken down into volatile and char in this process. The process of quenching, which applies fast cooling, comes after the gasification stage [47]. The next step is a cooling unit for the syngas, where heat is collected for syngas and may be utilised for a variety of things including electricity generation, hot water production, and space heating. The watergas shift reaction comes after the syngas cooling unit, which converts CO into CO2, the component that prior to the water gas shift process separates hydrogen sulphide (H2S) and carbon dioxide (CO2). After the hydrogen purification unit, there is an acid gas removal step where hydrogen is frequently separated from other gases using the pressure swing adsorption technique. The blue hydrogen production method is depicted in Figure 3.
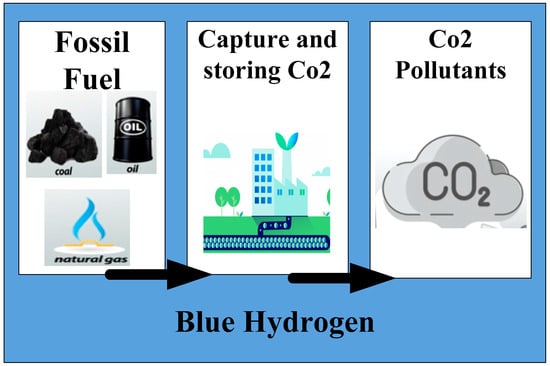
Figure 3. Schematic of Blue hydrogen production.
2.2. Purple Hydrogen Production Method
Purple hydrogen is produced using nuclear energy, and other forms of hydrogen may be produced using thermochemical processes thanks to the high temperatures of the nuclear reactor. The fission of uranium atoms produces nuclear energy. Heat from nuclear energy is used to create steam, which is then used in turbines to create electricity [48,49].
Nuclear power plants do not directly burn fuel; thus, they do not emit any greenhouse gases in that way. Because it can be controlled in the reactors used in nuclear power facilities, fission reaction is utilised. The fission process in the nuclear reactor serves as the heat source for the thermal energy in a nuclear power plant [50,51,52,53,54]. Nuclear reactor heat is used to make steam, which is subsequently utilised in a turbine to produce electricity using a generator similar to conventional stations that produce thermal energy. Nuclear energy is created by the nuclear reactor’s atoms splitting process, which transforms water into steam and drives a turbine to generate electricity. The most popular way to use this thermal energy is to turn a turbine to generate electricity, which may then be used to power fuel cells to create hydrogen [55,56]. Other ways to use this thermal energy include steam reforming techniques, water electrolysis for the creation of hydrogen, and thermochemical water splitting cycles.
2.3. Turquoise Hydrogen
Hydrocarbons split to generate turquoise hydrogen. This may be accomplished using a variety of methods. The approach that has advanced the most in research and is most likely to be commercialised is the plasma process for producing carbon black and hydrogen. Other techniques include cold plasma, methane catalytic conversion and molten metal pyrolysis via thermal splitting [57,58]. Hydrocarbons such as natural gas are utilised as feedstock and process energy for all of these operations, and electricity is the source of both. Methane splitting potentially requires 38 kJ/mol H2, while water electrolysis requires 285 kJ/mol H2 and steam-methane reforming requires 252 kJ/mol H2. Methane splitting requires high temperatures and heat losses [59,60].
2.4. Production of Grey Hydrogen
Grey hydrogen is the result of the steam reformation of coal or natural gas without the addition, usage, or storage of carbon. A by-product of other chemical processes produces more than 40% of grey hydrogen [61].
White hydrogen has also been unofficially coined by the North American Council for Freight Efficiency [62]. Grey hydrogen is mostly used in the petrochemical industry and in the production of ammonia [63]. The main disadvantage of grey hydrogen is due to the significant CO2 emissions that are generated during hydrogen synthesis, which are estimated to be around 830 Mt of CO2 yearly [64]. However, a tried-and-true technique that generates hydrogen at a fair price is natural gas steam reforming (SMR) without CCUS. During the procedure, the water is heated and natural gas is pre-treated. The methane is subsequently broken down into syngas in the reformer with steam.
Another important method of producing grey hydrogen is coal gasification, which is sometimes referred to as brown hydrogen in the literature. Since coal has the largest reserves of any fossil fuel in the world, this production method is also frequently used. China, in particular, generates a substantial quantity of hydrogen by coal gasification due to the high cost of natural gas and the abundance of coal reserves [59]. Four main types of coal, including lignite (low rank), sub-bituminous coal (low rank), bituminous coals (middle rank), and anthracites, are widely used as gasification feedstock (high rank) [61,62,63]. Figure 4 shows grey hydrogen production.
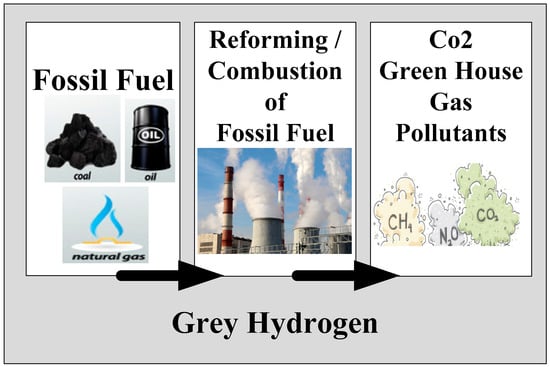
Figure 4. Schematic of Grey hydrogen production.
This entry is adapted from the peer-reviewed paper 10.3390/en16031141
This entry is offline, you can click here to edit this entry!