The term ‘cytokine storm’ (CS) applies to a pathological autoimmune reaction when the interactions that lead to cytokine production are destabilised and may even lead to death. CS may be induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. It is noteworthy that many of the criteria used to diagnose haemophagocytic lymphohistiocytosis (HLH) are described as COVID-19 mortality predictors. Cytokine storms are considered to be an important cause of death in patients with the severe course of SARS-CoV-2 infection. Due to the fact that pregnant women are in an immunosuppressive state, viral pulmonary infections are more perilous for them—possible risks include miscarriage, intrauterine growth restriction or birth before the term; sometimes ventilation support is needed. HLH should be considered in pregnant and puerperal women suffering from moderately severe to severe COVID-19 and presenting with: fever unresponsive to antibiotic therapy, cytopenia, hepatitis and hyperferritinaemia.
- cytokine storm
- COVID-19
- haemophagocytic lymphohistiocytosis
- pregnancy
- postpartum period
1. Introduction
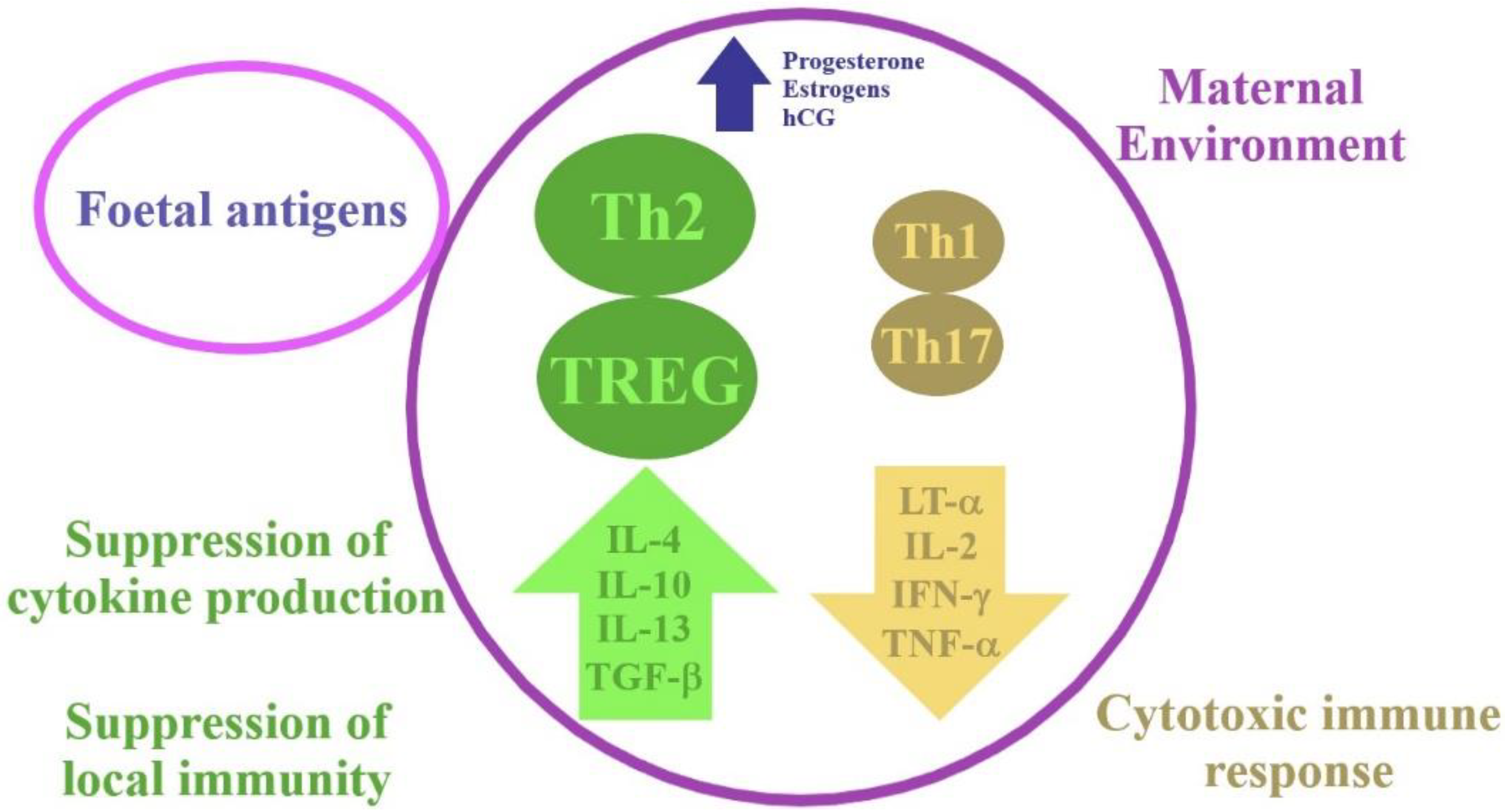
2. Physiological Changes in the Immune System during Pregnancy
3. Immunological Aspects in COVID-19 and Haemophagocytic Lymphohistiocytosis (HLH)
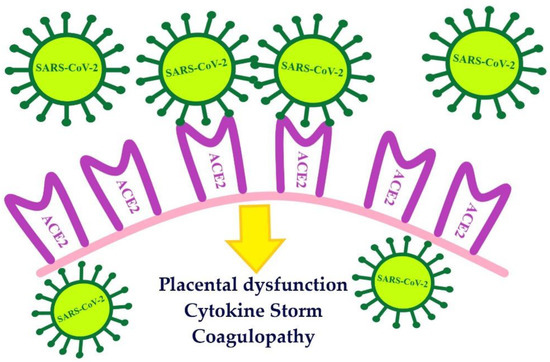
4. The Course of COVID-19 in Pregnant and Postpartum Women
5. The Course of HLH in Pregnant and Postpartum Women
Pregnancy is a physiological process, and the HLH syndrome is rarely diagnosed in pregnant women. Cytotoxic T cells, macrophages and NK cells play an essential role in HLH. Excessive activation and proliferation of T-cells and macrophages, and secretion of large amounts of cytokines, may be responsible for multiple organ failure, including the liver, brain and bone marrow, which can be potentially fatal [25]. The levels of cytokines such as TNF-α, IL-1β, IL-6, IL-10, and IFN-γ increase in HLH [26].
In order to diagnose HLH, either molecular diagnostics consistent with HLH must be performed or five of the eight diagnostic criteria for HLH must be fulfilled, i.e., splenomegaly, fever, cytopenia (affecting two or more of three lineages in the peripheral blood), hypofibrinogenaemia and/or hypertriglyceridaemia, elevated levels of ferritin, haemophagocytosis in the bone marrow/spleen/lymph nodes, low or absent NK cell activity and increased levels of soluble CD25 (interleukin [IL]-2 receptor), or both [27].
6. Associations between HLH and COVID-19
Recently, some similarity has been noticed between moderate and severe COVID-19 and HLH (Table 1).
| Moderately Severe to Severe COVID-19 | sHLH |
|---|---|
| prevalence in pregnant women—rarely diagnosed | |
| aetiology | |
| viral infection | viral infection in most cases |
| hyperferritinaemia | |
| marker of poor prognosis in COVID-19 patients | the most characteristic laboratory result of HLH |
| treatment | |
| treatment aimed at suppressing the cytokine storm | |
| high mortality | |
| increased levels of IL-6 | |
7. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/biom11081202
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Jamieson, D.J.; Honein, M.A.; Rasmussen, S.A.; Williams, J.L.; Swerdlow, D.L.; Biggerstaff, M.S.; Lindstrom, S.; Louie, J.K.; Christ, C.M.; Bohm, S.R.; et al. Novel Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009, 374, 451–458.
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112.
- Wadia, P.P.; Tambur, A.R. Yin and yang of cytokine regulation in solid organ graft rejection and tolerance. Clin. Lab. Med. 2008, 28, 469–479.
- Kroschinsky, F.; Stölzel, F.; von Bonin, S.; Beutel, G.; Kochanek, M.; Kiehl, M.; Schellongowski, P. Intensive Care in Hematological and Oncological Patients (iCHOP) Collaborative Group. New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit. Care 2017, 21, 89.
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41.
- Liu, D.; Li, L.; Wu, X.; Zheng, D.; Wang, J.; Yang, L.; Zheng, C. Pregnancy and Perinatal Outcomes of Women With Coronavirus Disease (COVID-19) Pneumonia: A Preliminary Analysis. AJR Am. J. Roentgenol. 2020, 215, 127–132.
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151.
- Narang, K.; Enninga, E.A.L.; Gunaratne, M.D.S.K.; Ibirogba, E.R.; Trad, A.T.A.; Elrefaei, A.; Theiler, R.N.; Ruano, R.; Szymanski, L.M.; Chakraborty, R.; et al. SARS-CoV-2 Infection and COVID-19 During Pregnancy: A Multidisciplinary Review. Mayo Clin. Proc. 2020, 95, 1750–1765.
- Liu, T.; Zhang, J.; Yang, Y.; Ma, H.; Li, Z.; Zhang, J.; Cheng, J.; Zhang, X.; Zhao, Y.; Xia, Z.; et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol. Med. 2020, 12, e12421.
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32.
- Mazzoni, A.; Salvati, L.; Maggi, L.; Capone, M.; Vanni, A.; Spinicci, M.; Mencarini, J.; Caporale, R.; Peruzzi, B.; Antonelli, A.; et al. Impaired Immune Cell Cytotoxicity in Severe COVID-19 Is IL-6 Dependent. J. Clin. Investig. 2020, 130, 4694–4703.
- Osman, M.; Faridi, R.M.; Sligl, W.; Shabani-Rad, M.T.; Dharmani-Khan, P.; Parker, A.; Kalra, A.; Tripathi, M.B.; Storek, J.; Cohen Tervaert, J.W.; et al. Impaired Natural Killer Cell Counts and Cytolytic Activity in Patients with Severe COVID-19. Blood Adv. 2020, 4, 5035–5039.
- Dimopoulos, G.; de Mast, Q.; Markou, N.; Theodorakopoulou, M.; Komnos, A.; Mouktaroudi, M.; Netea, M.G.; Spyridopoulos, T.; Verheggen, R.J.; Hoogerwerf, J.; et al. Favorable Anakinra Responses in Severe Covid-19 Patients with Secondary Hemophagocytic Lymphohistiocytosis. Cell Host Microbe 2020, 28, e1–e123.
- Obuchowska, A.; Kamiński, M.; Kimber-Trojnar, Ż.; Grzesik, P.; Standyło, A.; Turżańska, K.; Leszczyńska-Gorzelak, B. Haemophagocytic lymphohistiocytosis in pregnant and postpartum women. Wiad. Lek. 2020, 73, 1844–1847.
- Ramos-Casals, M.; Brito-Zerón, P.; López-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult haemophagocytic syndrome. Lancet 2014, 383, 1503–1516.
- Alcami, A.; Koszinowski, U.H. Viral Mechanisms of Immune Evasion. Trends Microbiol. 2000, 8, 410–418.
- Yongzhi, X. COVID-19-Associated Cytokine Storm Syndrome and Diagnostic Principles: An Old and New Issue. Emerg. Microbes Infect. 2021, 10, 266–276.
- Vietzen, H.; Zoufaly, A.; Traugott, M.; Aberle, J.; Aberle, S.W.; Puchhammer-Stöckl, E. Deletion of the NKG2C Receptor Encoding KLRC2 Gene and HLA-E Variants Are Risk Factors for Severe COVID-19. Genet. Med. 2021, 23, 963–967.
- Schwartz, D.A.; Graham, A.L. Potential Maternal and Infant Outcomes from (Wuhan) Coronavirus 2019-nCoV Infecting Pregnant Women: Lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses 2020, 12, 194.
- Amaral, W.N.D.; Moraes, C.L.; Rodrigues, A.P.D.S.; Noll, M.; Arruda, J.T.; Mendonça, C.R. Maternal Coronavirus Infections and Neonates Born to Mothers with SARS-CoV-2: A Systematic Review. Healthcare 2020, 8, 511.
- Karimi, L.; Makvandi, S.; Vahedian-Azimi, A.; Sathyapalan, T.; Sahebkar, A. Effect of COVID-19 on Mortality of Pregnant and Postpartum Women: A Systematic Review and Meta-Analysis. J. Pregnancy 2021, 2021, 8870129.
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320.
- Turan, O.; Hakim, A.; Dashraath, P.; Jeslyn, W.J.L.; Wright, A.; Abdul-Kadir, R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: A systematic review. Int. J. Gynaecol. Obstet. 2020, 151, 7–16.
- Park, H.S.; Kim, D.Y.; Lee, J.H.; Lee, J.H.; Kim, S.D.; Park, Y.H.; Lee, J.S.; Kim, B.Y.; Jeon, M.; Kang, Y.A.; et al. Clinical features of adult patients with secondary hemophagocytic lymphohistiocytosis from causes other than lymphoma: An analysis of treatment outcome and prognostic factors. Ann. Hematol. 2012, 91, 897–904.
- Cheng, J.; Niu, J.; Wang, Y.; Wang, C.; Zhou, Q.; Chen, Y.; Zhang, Y.; Lin, J.; Di, W. Hemophagocytic lymphohistiocytosis in pregnancy: A case report and review of the literature. J. Obstet. Gynaecol. 2020, 40, 153–159.
- Samra, B.; Yasmin, M.; Arnaout, S.; Azzi, J. Idiopathic Hemophagocytic Lymphohistiocytosis During Pregnancy Treated with Steroids. Hematol. Rep. 2015, 7, 6100.
- Henter, J.-I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131.
- Yildiz, H.; Vandercam, B.; Thissen, X.; Komuta, M.; Lanthier, N.; Debieve, F.; Dahlqvist, G. Hepatitis during pregnancy: A case of hemophagocytic lymphohistiocytosis. Clin. Res. Hepatol. Gastroenterol. 2018, 42, e49–e55.
- Hamizi, K.; Aouidane, S.; Belaaloui, G. Etoposide-based therapy for severe forms of COVID-19. Med. Hypotheses 2020, 142, 109826.
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768.
- Caricchio, R.; Gallucci, M.; Dass, C.; Zhang, X.; Gallucci, S.; Fleece, D.; Bromberg, M.; Criner, G.J.; Temple University COVID-19 Research Group. Preliminary predictive criteria for COVID-19 cytokine storm. Ann. Rheum. Dis. 2021, 80, 88–95.
- Wood, H.; Jones, J.; Hui, K.; Mare, T.; Pirani, T.; Galloway, J.; Metaxa, V.; Benjamin, R.; Rutherford, A.; Cain, S.; et al. Secondary HLH is uncommon in severe COVID-19. Br. J. Haematol. 2020, 190, e283–e285.
- Kyriazopoulou, E.; Leventogiannis, K.; Norrby-Teglund, A.; Dimopoulos, G.; Pantazi, A.; Orfanos, S.E.; Rovina, N.; Tsangaris, I.; Gkavogianni, T.; Botsa, E.; et al. Macrophage activation-like syndrome: An immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017, 15, 172.
- Charles, A.; Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001.
- van Eeden, C.; Khan, L.; Osman, M.S.; Cohen Tervaert, J.W. Natural Killer Cell Dysfunction and Its Role in COVID-19. Int. J. Mol. Sci. 2020, 21, 6351.
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474.
