1. The Skin
The skin is the largest organ of the body and performs different crucial functions [
17]. Being continuously exposed to external pathogens, it represents the first body’s barrier, and it is involved in key processes, such as temperature regulation, hydration, vitamin D synthesis, protection from physical and chemical dangerous
stimuli, and immune response [
18]. Skin is constituted by three main layers: the epidermis, the dermis, and the hypodermis. The epidermis is the uppermost layer and exerts sensitive and immunological roles. The dermis is the underlying part; it is connective tissue and supports the skin, contributing to its flexibility [
19]. The blood vessels transport nutrients and allow an inflammatory response, recruiting inflammatory cells. Below the dermis, the hypodermis is involved in thermoregulation and nutrition storage.
Given its essential functions, every interruption of the skin has to be considered dangerous and properly treated. Skin wounds, indeed, can potentially cause systemic inflammation and dangerous reactions, such as sepsis [
20]. Consequently, the wound healing process—to restore skin integrity—is essential [
21].
Wound Healing Phases
Wound healing is a complex and multifactorial process that involves different biological pathways [
22], including inflammation and redox regulation [
23]. Wound repair can be divided into four different but partially overlapping steps, namely haemostasis, inflammation, proliferation, and tissue remodeling [
24,
25].
Haemostasis. It typically begins immediately after an injury occurs: in a few minutes, a blood clot is formed in order to stop the bleeding. Platelets are key cells in this phase: they seal off the injured blood vessel—stopping the bleeding—and activate the chemotactic recruiting of inflammatory cells, such as macrophages and leukocytes. Moreover, platelets release cytokines and growth factors that, in turn, provoke the proliferation of smooth muscle cells and fibroblasts, starting the repair of the injured area, and promoting the inflammatory response [
26].
Inflammation. Inflammatory cells participate in microorganism killing, favoring successful healing [
27]. Furthermore, the recruited and activated immune cells start releasing a series of growth and haemostatic factors that, in turn, stimulate both the blood vessels repairing and the inflammatory response. This environment increases reactive oxygen species (ROS) production and, subsequently, leads to oxidative stress. The inflammatory phase generally follows and overlaps with the proliferative phase.
Proliferation. It is characterized by the macrophage switch from an inflammatory to a proliferative phenotype [
28]. Thus, the subsequent production of specific growth factors stimulates the proliferation of involved cellular types, forming a new granulating tissue on the wound surface. Meanwhile, the basal cells migrate to the surface to regenerate new epithelial tissue.
Tissue remodeling. Remodeling and maturation phases lead to the completion of the wound repair. During these phases, the components of the extracellular matrix are restored, and the proliferation is significantly reduced to the normal homeostatic equilibrium [
28]. Meanwhile, collagen is deposited on the wound site contributing to normal tissue restoration.
Most wounds end with a complete reformation of the corresponding normal tissue and generally heal in 4–6 weeks. However, a series of factors can affect the restorative process and end up in impaired healing by transforming the lesion into a chronic condition over time. Among them are the presence of vascular, metabolic, and autoimmune diseases, as well as age and ongoing drug therapy. Thus, the use of materials able to enhance wound healing has been shown to be effective in promoting tissue repair and avoiding scar tissue formation.
2. Wound Dressing
Wound dressing is a crucial issue in wound management that has a huge impact on health spending. Historically, wound dressing was intended as an inert barrier to prevent wound infection from external agents, and it was represented by gauze or tissue band-aids that did not actively change the wound’s status. For the first time in 1962, George Winter introduced the concept of wet dressing. He observed that covering skin wounds with polythene films accelerated the healing process with respect to air-exposed injuries [
29,
30]. This evidence showed that keeping the injury site moisturized could enhance tissue repair by promoting cell migration [
31]. These results prompted the interest of the scientific community in developing smart wound dressing systems over the years. To date, several materials have been explored and exploited to get dressings that can actively participate in the wound-healing process [
32,
33]. The new generation of wound dressings includes devices that can sense and respond to the wound environment’s
stimuli [
33]. Recently,
stimuli-responsive hydrogels have found a large application in the treatment of chronic wounds, such as diabetic ones. The latter still represents a serious challenge: about 20% of diabetics worldwide suffer from poor wound healing [
34] with severe associated complications and high costs.
Smart materials have been proven to be very effective in improving the repair of these wounds due to their capacities to change mechanical properties, swelling ability, and hydrophilicity in response to different
stimuli, such as temperature, pH, light, and so on [
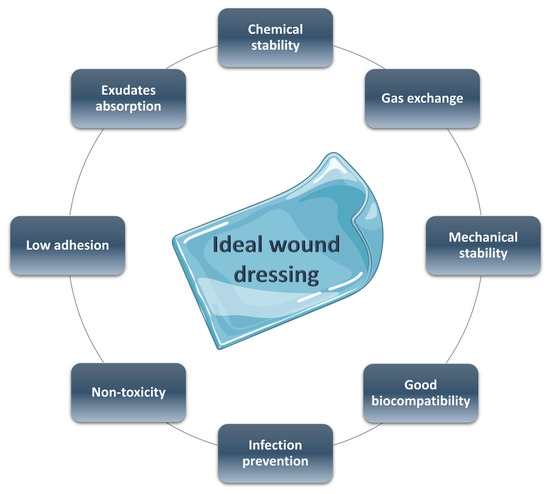
35]. An ideal wound dressing needs the characteristics reported in
Figure 1: (a) good biocompatibility, (b) adequate physical and mechanical properties, (c) surface microstructure in order to permit cell adhesion and differentiation, (d) good moisture adsorption to guarantee the necessary wet environment, (e) low adhesion to the wound tissue to a facile and non-painful removing, (f) non-toxicity, (g) optimal gas exchange between the damaged tissue and the environment, and (h) antibacterial properties [
36,
37].
Figure 1. Ideal features of a wound dressing.
3. Stimuli-Responsive Systems
3.1. pH-Responsive
The skin’s physical structure, fundamental for the performance of its functions, consists of two main layers: the epidermis—with the stratum corneum—principally constituted by keratinocytes, and the dermis, composed of fibroblasts, collagen, and other extracellular components. Furthermore, various skin appendages, such as eccrine and sebaceous glands, exert specific functions. The former secrete sweat in response to different
stimuli; the sebaceous glands produce sebum, which lubricates the skin, also maintaining its surface at a pH value of about five [
64]. This resulting acidic pH also referred to as acid mantle, represents a key factor in keeping a healthy skin environment since it contributes to barrier homeostasis and integrity and antimicrobial defense [
65]. In addition, in this acidic condition, antimicrobial peptides, an important component of innate immunity, are able to work the most [
66].
In light of the above, it is clear that pH is crucial in maintaining the correct function of the skin, and it is a strictly regulated factor. pH, indeed, changes in the different layers, increasing from the surface to the deeper ones. Furthermore, it varies in the different phases of wound healing [
67,
68]. pH alterations can inhibit necessary enzymes, impairing physiological repair. Still, bacterial infection and colonization—typical of chronic wounds—are also affected by topical pH variations. It is different depending on the skin’s status: healthy skin presents a slightly acidic value (5–6), acute wounds have a pH around 7.4, while chronic ones have a more alkaline pH between 7.3 and 10 (partly due to the proliferating bacterial colonies) [
69,
70,
71].
Based on these findings, several pH-responsive materials have been explored for controlled drug release in both acute and chronic wounds with the aim of improving the healing process. Most of these responsive systems are hydrogel-based since they exploit the numerous advantages peculiar to these materials [
6,
36]. The active molecules can be loaded into the polymeric matrix and then released under specific conditions.
In a very recent study, Bostanci and collaborators presented photocrosslinked hydrogels based on methacrylated forms of pectin and gelatin (PeMA and GelMA, respectively) for pH-dependent release of curcumin, used as antimicrobial agents. In this system, the presence of carboxylic groups on both polymers as well as the pH-dependent solubility of curcumin, resulted in its modulated release, exerting antibacterial activity and allowing a high cell viability [
72].
In 2021 Li et al. [
73] developed a pH-responsive injectable hydrogel consisting of N-carboxyethyl chitosan, aldehyde hyaluronic acid, and adipic acid dihydrazide for the treatment of diabetic foot ulcers. The release of insulin, encapsulated in the hydrogel dressing, decreased glucose levels, promoting wound healing. The prolonged pH-driven release of insulin is allowed by the presence of acylhydrazone and imine bonds, which undergo rupture in acidic pH environments and significantly affect the hydrogel networks, favoring the drug release.
Lastly, Ahmadian and co-workers presented a multifunctional gelatin-tannic acid (GelTA) hydrogel as a promising candidate for the treatment of chronically infected wounds. They proposed a safe, reproducible one-pot synthesis of a natural hydrogen-bonded hydrogel with high biocompatibility and pH-dependent release of embedded antioxidants. In addition, the hydrogel showed excellent therapeutic effects on wound repair in vivo, promoting cell migration and proliferation, as well as collagen production, being suitable for wound healing applications [
74].
3.2. ROS-Responsive
ROS are crucial regulating factors of the healing process [
76], improving physiological skin repair. ROS, indeed, participate in oxidative bacterial killing promoting angiogenesis and re-epithelialization at the wound site. In addition, ROS acts as secondary messengers inducing the activation of several transcription factors, such as nuclear factor kappa B (NF-kB), which is one of the main actors of inflammation. Nevertheless, excessive levels of ROS cause oxidative damage, impairing the correct healing; this phenomenon is especially observed in chronic wounds where a prolonged state of oxidative stress occurs [
77]. Targeting oxidative stress in chronic wounds by restoring a redox equilibrium has been proven effective in improving proper wound repair [
78].
The well-known property of selenium (Se) to undergo a hydrophobic-hydrophilic transition under oxidation is exploited in Se-containing block copolymers [
79]. When oxidized, indeed, Se is converted into more polar selenoxides and/or selenones. Moreover, compared to other chalcogens, Se-based materials are particularly advantageous due to the lower bond energy of C–Se (244 kJmol
−1) and Se–Se (172 kJmol
−1). In addition, the Se-Se bond is susceptible both to reducing and oxidizing conditions: it is cleaved and oxidized to seleninic acid in the presence of oxidants and reduced to selenol in a reducing environment. This responsive disassembling of Se-containing block copolymers makes them suitable biomaterials for controlled drug release.
Ma et al. developed ROS-responsive selenium-containing block copolymer for drug delivery applications. They prepared a diselenide containing polyurethane (PUSeSe) block copolymer finally terminated by PEG (PEG-PUSeSe-PEG) incorporating fluorescent Rhodamine B as a drug model. The PEG-PUSeSe-PEG showed to be disassembled—releasing their cargo—in a mild oxidative environment (0.1%
v/
v H
2O
2) [
80]. Moreover, the material was also responsive to reductant
stimuli (GSH 0.01 mg/mL). This characteristic confers key amphiphilic properties that are useful in wound healing acceleration.
ROS-responsive biomaterials that are able to scavenge ROS present a real potential in diabetic wound treatment. Diabetic wounds are a chronic health issue that leads to hindered skin regeneration.
Zhu et al. prepared an injectable, amphiphilic block copolymer, including poly(ethylene glycol)-b-poly(propylene sulfide) (PEG-PPS), able to self-assemble in aqueous solution becoming a stable hydrogel [
81]. This star-PEG-PPS scaffold fills the entire wound bed, providing structural support for cellular infiltration and tissue regeneration. In their system, oxidized polypropylene sulfide acts as a reactive oxygen species (ROS) quencher in reactive wounds, favoring the repair.
Zhao and collaborators have proposed a poly (vinyl alcohol) (PVA)-based, ROS-responsive hydrogel to promote healing in diabetic wounds [
82]. Due to the insertion of a ROS-responsive linker, namely N1-(4-boronobenzyl)-N3-(4-boronophenyl)-N1, N1, N3, N3-tetramethylpropane-1, 3-diaminium (TPA); they obtained a ROS-responsive degradation of the final system, with the consequent controlled release of the embedded active molecules. The obtained hydrogel showed an effective ROS-scavenging activity by decreasing ROS levels.
In a recent study, Hu et al. [
83] proposed a ROS-responsive hydrogel-based nanocomposite with antibacterial properties to use in infected wounds. They prepared a polymer constituted of polyacrylic acid (PA) containing AgNPs and Fe
2+/Fe
3+-contained polyglutamic acid (PG). The resulting hydrogel, named PAAg-PGFe, showed a semi-solid status in physiological conditions, while the exposure to H
2O
2 caused hydrogel breakage with consequent exerting of antibacterial activity. Furthermore, it exhibited no toxicity in mammalian cells in vitro, whereas it demonstrated quick wound healing and pathogen removal in vivo experiments.
In a very interesting study, carboxymethyl chitosan (CMCTS)-based hydrogel crosslinked by a ROS-sensitive linker (thioketone group, Tk) and loaded with curcumin (Cur) Cur@CMCTS-Tk was explored as an effective wound dressing. This system exhibited topical and controlled release of Cur, a potent antioxidant, removing ROS in the wound site and showing a potential application in burn wound treatment both in vitro and in vivo [
84].
Alternately, double-network (DN) hydrogels as a double-responsive drug delivery system for chronic wound treatment were proposed [
85]. The DN system was obtained by incorporating polyacrylamide (PAM) into catechol chitosan (C-CS) hydrogel, crosslinked, using Bis(acryloyl)cystamine (BAC) and Cystamine (Cys), respectively. PAM enhanced the system’s mechanical properties, while Cys disulfide bonds made the hydrogel network ROS-responsive. Moreover, catechol groups enhanced tissue and cell adhesion, promoting wound healing. In addition, embedded pH-responsive nanoparticles—prepared by acetalized cyclodextrin—were used for the pH-dependent release of anti-inflammatory molecules.
3.3. Light-Sensitive
Recently,
stimuli-responsive hydrogels have emerged as promising smart wound dressings. Photo-responsiveness is particularly advantageous since the
stimulus of biomaterials is constituted by light. Light is a non-invasive form of energy that does not require chemical agents to exert its effect. It can be finely modulated depending on exposure time and intensity, as well as on the selected wavelength [
86]. Thus, the combination of light energy and hydrogel responsiveness led to the development of several efficient systems for wound treatment. A recent and comprehensive review of photoactive hydrogels for wound healing improvement has been published by Maleki et al. [
87].
Shi and collaborators just presented a new dual light-responsive cellulose nanofibril (CNF)-based in situ hydrogel wound dressing (CNF-DLRIHWD). In the first place, CNFs were grafted with antibacterial agents polyaminopropyl biguanide and Protoporphyrin IX (PAPB and PpIX, respectively). CNFs were then integrated with Prussian blue nanoparticles (PBNPs), Pluronic
® F127 (F127), and hydroxypropyl methylcellulose (HPMC). This system contributes to wound healing by exerting an excellent antimicrobial effect, joined with photodynamic and photothermal therapies. Moreover, the resulting nanocomposite was shown to be able to counteract bacterial biofilms, promoting the repair of infected chronic wounds [
88].
Likewise, Yang et al. [
89] developed a dual-functional system with antibacterial and wound-healing dressing features. A dodecyl-modified chitosan hydrogel, linked to dialdehyde-functionalized PEG via a Schiff base reaction, was added with WS2 nanosheets—a photothermal agent—and loaded with ciprofloxacin—an antimicrobial drug. Under the irradiation of near-infrared (NIR) light, WS2 generates heat, providing a photothermal treatment and photo-response drug therapy simultaneously. The antibiotic, released in a spatially and temporally controlled mode, leads to bacterial death and eliminates the inflammatory response, showing a good anti-oxidation activity that, in turn, promotes wound healing.
Recently, Li et al. [
90] proposed a NIR-responsive system consisting of polydopamine-hyaluronic acid (PDA-HA) hydrogel-loaded calcium peroxide-indocyanine green added to lauric acid and manganese dioxide (CaO
2-ICG@LA@MnO
2) nanoparticles. In detail, a core shell, in which CaO
2 is partially linked with ICG, is covered by LA and then combined with MnO
2. By irradiating the system with NIR laser light, an on/off release of O
2 is obtained. In detail, the induced photothermal effect causes the LA to melt at 40 °C. Consequently, CaO
2, now in an aqueous environment, is hydrolyzed to H
2O
2, which in turn produces O
2. In this system, LA hydrophobicity restrains the O
2 release, while MnO
2 is the reaction catalyst and MnO
2 is the reaction catalyst.
3.4. Glucose-Responsive
Glucose-responsive hydrogels find a specific application in diabetic chronic wound treatment. In diabetics, skin repair is often impaired due to elevated blood glucose levels [
91,
92]. Hyperglycemia, indeed, induces chronic inflammation, altered angiogenesis, and augmented production of glycation end products and strong oxidative stress conditions [
93]. All these co-existent factors contribute to a compromised wound healing process characterized by prolonged inflammation and reduced re-epithelization [
94,
95,
96]. Diabetic wounds, indeed, still represent a health care challenge: the diabetic foot ulcer causes disability, amputation, and ultimately death if it is not properly treated [
97]. Due to this, several hydrogel-based wound dressings have been proposed for improved repair [
98].
Xu and collaborators proposed a novel glucose-responsive hydrogel with antioxidant properties to enhance wound repair in these wounds [
60]. They grafted gallic acid (GA) on the surface of chitosan (CS) chains and then embedded it in a PEGDA hydrogel matrix. Moreover, they modified polyethyleneimine (PEI) NPS with glucose-sensitive phenylboronic acid (PBA) and loaded the resulting NPs with insulin. The NPs were linked to the CS-GA chains through the borate bond between the GA hydroxyl groups and phenylboronic acid groups. The resulting platform was able to release insulin in hyperglycemic conditions. In addition, the antioxidant activity, due to the embedded polyphenol, improved the repair by re-establishing a redox balance.
Similarly, Chen et al. recently presented a device for the controlled release of (−)-epigallocatechin-3-gallate (EGCG), a natural polyphenol with proven antioxidant activity, in the presence of high glucose levels. The system was prepared by modifying Gelatin methacryloyl (GelMA) with 4-carboxyphenyboronic acid (CPBA). Then, the inclusion of EGCG allowed the formation of glucose-responsive boronic ester bonds between the PBA groups of the matrix and the ortho-dihydroxy groups of EGCG. Due to this peculiar interaction, the resulting material showed an EGCG sustained release in a glucose-responsive manner, accelerating wound healing by eliminating excessive ROS in the wound site [
99]. In vitro and in vivo studies confirmed that they had a high grade of biocompatibility and no toxicity paving the way to a possible clinical application.
Yang and collaborators proposed a GOx-containing, multifunctional metalorganic drug-loaded hydrogel (DG@Gel). When applied to a diabetic wound, the GOx causes a decrease in glucose levels in the wound microenvironment by converting glucose into gluconic acid and hydrogen peroxide. This reaction provokes a decrease in pH levels that—in turn—leads to the material’s swelling with a consequent release of loaded active molecules. Due to this, the designed system presented a synergistic effect in diabetic wound healing acceleration, both in vitro and in vivo experiments [
100].
3.5. Thermosensitive
According to the classification of Zamboni et al. [
101], thermo-responses are part of the second-generation hydrogels and show numerous advantages in controlled release systems. They are usually crosslinked by non-covalent interactions, and their physical state is temperature driven. This can induce the sol–gel transition, allowing the perfect adaptability of the gel on the wound site, even for the injectable type avoiding surgical implantation. Incorporating the drug in a flow state guarantees a homogeneous dispersion; meanwhile, the rapid gel formation—via sol–gel transition, often occurring at physiological temperature—prevents an initial burst release, providing a sustaining delivery. Thermo-responsive hydrogels can swell/shrink according to the environmental temperature [
102], with related changes in volume due to the hydrophobic/hydrophilic functional groups present in the gels’ chemical structure.
Among synthetic temperature-sensitive hydrogels, the ones based on poly (N-isopropylacrylamide) (PNIPAAM) are the most extensively studied [
103]. They have a lower critical solution temperature (LCST), close to the body temperature (32 °C). Above this value, the hydrogen bonds break, and the solution turns into a gel-like state due to hydrophobic interactions [
104]. A hybrid hydrogel, obtained by a combination of a short peptide (I3K) with PNIPAM [
105], resulted in a system—I3K self-assembled fibrils entangling with PNIPAM—forming a 3D network. An antibacterial peptide G(IIKK)3I-NH2, loaded as a model drug, assessed the sustained and linear release in the aqueous environment at higher temperatures in the wound site. Indeed, positive controlled thermo-responsive hydrogels (
i.e., PNIPAM) allow a more rapid drug release at increased temperature—characteristic of the chronic wound’s inflammatory state—and slower delivery at a lower temperature.
Analogously, natural hydrogel chitosan (CTS)-based systems have been deeply investigated, especially due to their excellent biocompatibility. In particular, the presence of β-glycerolphosphate (β-GP) is reported to allow the sol–gel transformation in CTS-based hydrogel at physiological temperature (37 °C) [
106]. In a recent study [
107], a β-GP-CTS-based thermo-responsive polymer led to a significant decrease in bacterial population in infected wounds, in vivo experiments with extensively drug-resistant (XDR). Bacteria were clinically isolated from burn patients, accomplished with an acceleration of wound healing, re-epithelialization, and wound closure.
However, the temperature guide, while conferring the dynamic behavior, makes these hydrogels potentially unstable, as they could turn into the liquid phase at temperatures lower than the LCST or higher than the upper critical solution ones (UCST), failing the sustained release. Therefore, although they are widely applied as thermo-responsive polymers, their use can be affected by rapid dissolution in aqueous solutions, which strongly limits a prolonged release.
To overcome this limit, in a recent work, Yan and collaborators developed a hydrogel composed of poly(Nisopropylacrylamide166- co-n-butyl acrylate9)-poly(ethylene glycol)-poly(N-isopropylacrylamide166-co-n-butyl acrylate9) copolymer and silver-nanoparticles-decorated reduced graphene oxide nanosheets [
108]. The inorganic/polymeric dual network confers to the system stability with respect to the sol–gel transition, even at temperatures lower than LCST. The stable wound dressing activity is detected in the healing of a methicillin-resistant Staphylococcus aureus-infected skin lesion.
This entry is adapted from the peer-reviewed paper 10.3390/gels9060451