Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Sentinels are organisms whose characteristics (including health status) change due to acute or chronic effects in a given environment that can be evaluated (monitored) through serial surveillance.
- sentinels
- dogs
- cats
- environmental pollution
- indoor pollution
1. Introduction
Evidence that living beings can be natural sentinels of biological risks and environmental pollutants has been recognized for centuries [1]. In their book, Natterson-Horowitz and Bowers [2] point out that “In a world where no creature is truly isolated and disease spreads as fast as airplanes can fly, we are all canaries, and the entire planet it’s our coal mine”. Many birds, fish, wild and domesticated terrestrial mammals, and pets are valuable indicators of environmental pollution, displaying early warnings of exposure to a contaminated environment before humans are affected [3].
Sentinels are organisms whose characteristics (including health status) change due to acute or chronic effects in a given environment that can be evaluated (monitored) through serial surveillance [4][5][6][7]. Samples can be collected routinely, at random, or at predetermined intervals, and analyzed to identify potential health hazards to humans and other animals [4]. There are many criteria according to which a species should be considered a sentinel. In the first place, the sentinels must occupy a large area geographically close to human settlements and humans; their biology, sensitivity to pollutants, and bioaccumulation capacity have to be known [5]. They must have at least the same sensitivity to poisoning as humans, with similar physiology; their life span must be long enough to show the effects of not only the acute but of chronic exposures too, and the biological or clinical effects must develop early and be comparable to those in humans. The exposure pathway has to be similar to that of humans, which is feasible for companion animals sharing the same environment with their owners [4]. Among the phenotypic characteristics, the animals’ size is essential because of the sufficient amounts of tissue are needed for analysis, but other aspects, such as age or gender, must also be considered [6].
By testing and monitoring pets, we can detect early the presence and impact of pollutants for this information to be used to minimize the adverse effects on human health [7]. Thus, companion animals can literally be considered “sentinels” of environmental pollution [8].
The definition of sentinel organisms many times overlap with that of biomonitors, although the latter term may be considered broader. A biomonitor is an organism (or part of an organism or a community of organisms) that contains information on the quantitative aspects of the quality of the environment [9]. In this sense, sentinels are biomonitors too. Yet, active biomonitoring has a more intentional sense when, for example, biomonitors bred in laboratories are placed in a standardized manner in a certain environment to gather information. By contrast, especially when the term ‘natural sentinels’ is used, the organisms already present in the environment are monitored.
2. Pets as Sentinels for Environmental Pollution
2.1. Pets as Sentinels for Asbestos Fibers and Heavy Metals in the Environment
The usefulness of animals in the role of sentinels has been recognized more than a quarter-century ago. As the National Research Council of the United States devised [10] in 1991, the biologic effects of suspected toxic substances can be evaluated in animals kept in their natural habitat (including human homes) to assess the intensity of exposures, measure the effects of chemical mixtures, and determine the results of low-level exposures over a long period. Even more, observing the prevalence and incidence of certain pet pathologies reveal patterns that show the distribution of pollution in the area [11].
The harmful effects of metalloids and heavy metals are well described, and their association with specific diseases is as well, both in humans and companion animals (Table 1, Figure 1). The harmful health impact of these pollutants is similar, regardless the species exposed [12].
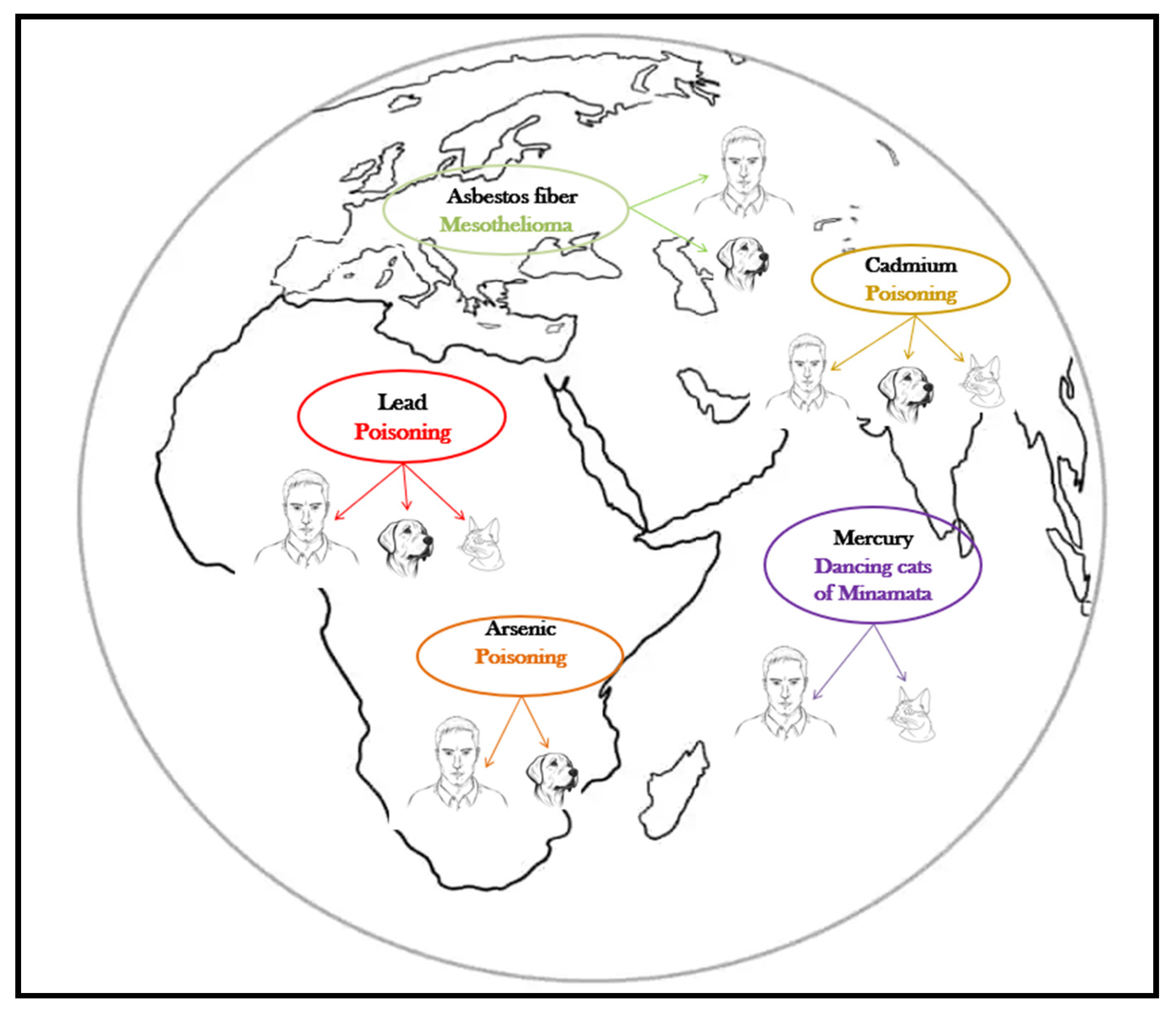
Figure 1. Pets, sentinels of environmental heavy metal/metalloid pollution.
Table 1. Human and small animal pathologies promoted by metalloid or heavy metal toxic exposure.
| Heavy Metal/Metalloid | Pets | Effects in Pets | References | Effects in Humans | References |
|---|---|---|---|---|---|
| Asbestos | Dogs | Canine malignant mesothelioma Pleural mesothelioma (more frequently than pericardial and peritoneal)—poor prognostic Pleural effusions, especially in old dogs, with males more prone than females |
[13] [14] [15] |
Human mesothelioma Pleural mesothelioma (more frequently pleural effusions) Peak incidence occurs in the 5th and 6th decades of life |
[16] [17] [18] |
| Chromium (Cr) | Dogs | Cardiac impairment, oxidative damage, altered ATP-aze content | [19] | Airway irritation, obstruction, and cancers Allergic dermatitis Dose dependent renal damage Gastrointestinal and hepatic impairment Hypoxic myocardiac changes Intravascular hemolysis |
[20] |
| Arsenic (As) | Dogs Cats |
Ulcerative dermatitis Myocarditis Bladder cancer Chronic renal failure |
[21] [22] |
Hyperpigmentation and keratosis Ischemic heart diseases Renal diseases Bladder cancer, skin, lungs, liver, kidney cancer Kidney damage |
[23] [24] [25] |
| Cadmium (Cd) | Dogs Cats |
Disrupted male reproduction Impaired pancreatic function Decreased bone-formation rate Chronic renal failure |
[26] [27] |
Altered male reproduction (hormonally and functionally) Disturbed calcium metabolism: osteopetrosis, osteomalaecia Itai-Itai disease (renal tubular dysfunction and osteomalaecia) Kidney failure |
[28] [29] [30] |
| Lead (Pb) | Dogs Cats |
Functional forebrain dysfunction and cortical blindness Anemia Epileptic seizures Bone sclerosis Chronic myocarditis chronic Renal failure |
[31] | Central and peripheral nervous system impairment Hematopoiesis problems, microcytic anemia Gastrointestinal disturbances Renal failure. |
[32] [33] [34] |
| Mercury (Hg) | Cats | Similar neurological symptoms to Minamata disease: ataxia, weakness, loss of balance, and motor incoordination | [35] [36] |
Neurological symptoms: uncontrollable tremors, muscular incoordination, slurred speech, partial blindness | [37] |
Canine mesothelioma is described as being linked to lifestyle, diet, and asbestos exposure, but the most cases have occurred in canine companions whose owners worked in environments with asbestos or used flea repellents in which the talc was contaminated with asbestos [38]. The human and canine malignant mesotheliomas are clinically and morphologically similar [39], but the latency period is much shorter in dogs (8 years) than in humans (up to 20 years) [38]. As for the effects of acute asbestos exposure, in a 15-year surveillance study in search-and-rescue dogs Otto et al. [40] reported no difference in the cause of death of dogs exposed to several classes of toxicants (including heavy metals and asbestos, during deployment at terrorist attack sites) compared to unexposed dogs.
Lead (Pb), cadmium (Cd), arsenic (As), and mercury (Hg) are all heavy metals/metalloids with adverse health effects, and their latency period is also shorter in pets compared to humans [41]. The absorption, metabolization, transformations, and toxicity of these substances in pets is the most similar to that of young children, which makes companion animals even more valuable in early detection [42]. In a 2005 study, Park et al. [43] concludes that dogs can be considered biomonitors of the environmental quality assessment for cadmium, lead, and chromium contamination, and at least for lead and cadmium cats showed the same conclusive results (Table 2).
Table 2. Average values of heavy metals found in the blood and certain organs of clinically healthy † pets from different polluted geographical areas.
| Country | U.M. | Heavy Metals (Mean ± SD) | Analyzed From | No. of Samples | Species | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pb | Cd | Cr | Hg | As | ||||||
| Korea | μg/mL | 0.68 ± 0.19 | 0.21 ± 0.01 | 0.66 ± 0.15 | 1.10 ± 0.49 | - | Serum | 204 | Dogs | [43] |
| Zambia | μg/L | 271.6 ± 226.9 | 1.5 ± 1.6 | 67.2 ± 75.4 | - | 5.2 ± 4.5 | Blood | 120 | Dogs | [44] |
| Italy (Campania) | mg/kg | 0.321 ± 0.198 | 0.093 ± 0.079 | - | 0.054 ± 0.044 | - | Liver | 38 | Dogs | [45] |
| 0.293 ± 0.231 | 0.259 ± 0.238 | - | 0.040 ± 0.021 | - | Kidney | |||||
| Italy (Naples) |
mg/Kg | 0.256 ± 0.130 | 0.098 ± 0.063 | - | - | - | Liver | 290 | Dogs | [46] |
| 0.147 ± 0.081 | 0.302 ± 0.212 | - | - | - | Kidney | |||||
| 0.268 ± 0.107 | 0.101 ± 0.054 | - | - | - | Liver | 88 | Cats | |||
| 0.189 ± 0.102 | 0.355 ± 0.144 | - | - | - | Kidney | |||||
| Italy (South Sardinia) | ng/mL | 81.4 ± 16.6 | 52.2 ± 14.0 | - | - | 139 ± 39 | Ovaries | 26 | Cats | [47] |
| 20.4 ± 3.6 | 19.7 ± 4.0 | - | - | 21.7 ± 4.2 | Ovaries | 21 | Dogs | |||
| Italy (North Sardinia) | ng/mL | 51.1 ± 17.9 | 26.4 ± 5.5 | - | - | 107 ± 61 | Ovaries | 14 | Cats | |
| 12.2 ± 5.2 | 12.2 ± 1.8 | - | - | 21.8 ± 3.9 | Ovaries | 24 | Dogs | |||
| Poland | 2.829 ± 3.490 ** 1.55 ± 1.71 ** |
0.105 ± 0.067 ** 0.096 ± 0.074 ** |
0.0020 ± 0.0013 * 0.0027 ± 0.0022 ** |
Cartilage and compact bone Spongy bone |
24 | Dogs | [48] | |||
| Poland | μg/mL | 0.419 ± 0.027 | 0.302 ± 0.049 | 0.244 ± 0.016 | - | 0.637 ± 0.277 | Serum | 48 | Dogs | [49] |
| Spain | μg/mL | 0.55 ± 0.60 | - | 2.73 ± 2.54 | 0.24 ± 0.22 | 1.86 ± 1.44 | - | 42 | Dogs | [50] |
| Jamaica West Indies | μg/mL | 2.83 ± 0.40 | - | - | - | - | Serum | 63 | Dogs | [51] |
| New Zealand | μg/mL | 0.23 ± 0.66 | - | - | - | - | - | 271 | Dogs | [52] |
| 0.21 ± 0.05 | - | - | - | - | - | 113 | Cats | |||
† One of the referenced papers [46] reports the heavy metal concentrations found in the tissues of animals deceased in an incident. U.M.: unit of measurement; * lower values than in humans; ** higher values than in humans.
Although the concentration of heavy metals can be measured in various samples, the liver and kidney tissue are preferred, especially for cadmium and lead exposure [45][46]. Once absorbed, lead is redistributed in the soft tissues [48], but most of it (90%) is accumulated in bones and teeth [48], with a half-life of 25 years in humans [53].
The benefits of using animal sentinels to evaluate environmental lead pollution have been recognized for more than 40 years [54]. This is extremely relevant, especially when pets live in proximity to children. Preventing exposure to lead during nervous system development is very important because of the damage this heavy metal can cause [55]. The tremendous benefit of this approach is that the blood tests of sentinels can provide early warnings without having to analyze a child’s brain [4]. As early as 1976, the comparative analysis of serum samples collected from 119 children and 94 dogs from suburban Illinois demonstrated that canine companions can be considered sentinels of lead poisoning in children [54]. The presence of lead in the blood of pets may indicate potential exposure of humans, especially of young children, to the polluted environment is also the conclusion reached by Berny et al. [56]. For possible lead contamination in children, the prediction is based on canine and feline serum values which exceed the physiologic threshold of the species 2–4 times [2][57]. However, for the results to be conclusive, variables such as sex, age, and behaviors influencing exposure of the companion animals must be taken into consideration. Although the blood levels of lead are usually higher in males, Toyomaki et al. [44] reports for one of their research areas a significantly higher lead contamination of female dogs, especially in the older ones. The authors’ supposition is that male dogs may wander more (thus leaving the polluted areas), while the females stay for longer while raising their puppies in the same (polluted) place.
After the period in which the sentinel role of companion animals for environmental asbestos and heavy metal pollution detection was proven by testing their blood and especially tissues (to verify chronic exposure) illustrated by many reports (Table 2), the research continued to find more non-invasive approaches [58]. In this context, Petrov et al. [59], and Nikolovski and Atanaskova [60] believe that using hair as a testing matrix brings significant advantages in terms of ease of collection and a more animal welfare friendly attitude compared to tissue sampling. As Table 3 shows, the concentration of several heavy metals has been tested in different countries by analyzing hair samples of the companion animals exposed to these pollutants.
Table 3. Mean values of heavy metals obtained by testing pet hair samples.
| Country | UM | Heavy Metals (Mean ± SD) | AM | No. of Samples |
Species | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pb | Cd | Cr | Hg | As | ||||||
| Macedonia | μg/kg | |||||||||
| Veles | 930.15 ± 516.03 | 54.28 ± 12.77 | - | - | - | AAS | 11 | Dog | [59] | |
| Bitola | 715.66 ± 293.80 | 42.65 ± 25.41 | - | - | - | 22 | Dog | |||
| Prilep | 525.63 ± 253.91 | 27.82 ± 8.31 | - | - | - | 11 | Dog | |||
| Macedonia | μg/kg | |||||||||
| Delcevo | 579 ± 478.29 | 68.57 ± 59.95 | - | - | - | AAS | 18 | Dog | [60] | |
| Probistip | 1061.38 ± 564.02 | 26.86 ± 23.30 | - | - | - | 20 | Dog | |||
| Veles | 1099.02 ± 593.01 | 171.54 ± 179.53 | - | - | - | 17 | Dog | |||
| Prilep | 370.57 ± 288.39 | 21.65 ± 10.64 | - | - | - | 18 | Dog | |||
| Bitola | 687.05 ± 482.82 | 66.04 ± 73.78 | - | - | - | 21 | Dog | |||
| Australia | mg/kg−1 DW | 1.19 ± 3.11 | - | 0.85 ± 1.42 | 0.13 ± 0.11 | 0.08 ± 0.06 | AAS | 36 | Dog | [61] |
| Argentina | mg/g DW−1 | - | - | - | - | 24 ± 2 | TXRF technique | - | Dog | [62] |
| Portugal | μg/g−1 | 24 ± 2.4 | TXRF | 50 | Dog | [63] | ||||
| Portugal | ng/g−1 | - | - | - | 24.16–826.30 | TXRF technique | 26 | Dog | [64] | |
| Poland | mg/kg−1 | - | - | - | 0.025 ± 0.020 | AAS | 85 | Cat | [65] | |
| Iran | ng/g DW | - | - | - | AAS | 40 | Wild cats | [66] | ||
| North-west | - | - | - | 735 ± 456 | ||||||
| North | - | - | - | 568 ± 381 692 ± 577 |
||||||
| Center | - | - | - | 1303 ± 1306 376 ± 162 |
||||||
| North-east | - | - | - | 1517 ± 1888 | ||||||
| West | - | - | - | 231 ± 89 | ||||||
| Japan | ppm | - | - | - | 7.40 ± 2.93 | AAS | 12 | Male cat | [67] | |
| - | - | - | 7.45 ± 1.28 | 29 | Female cat | |||||
| - | - | - | 0.99 ± 0.23 | 16 | Male dog | |||||
| - | - | - | 0.66 ± 0.10 | 18 | Female dog | |||||
| Alaska | ng/g | 1822.4 ± 1747 | - | Sled dog | [68] | |||||
UM—unit of measurement; DW—dry weight; AAS—atomic absorption spectrometry; AM—analysis method; TXRF—total reflection X-ray fluorescence analysis.
While testing canine hair, Jafari [61] observed a strong positive linear correlation between the contamination of the soil and the hair with chromium, copper, lead, and mercury, and to a lesser extent with zinc, and a negative correlation with arsenic. Promising results were obtained by testing feline hair too, for environmental lead pollution [69]. Aeluro and Kavanagh [69] found higher lead concentrations in the hair of female cats than of the males, but the authors report that these animals were more exposed (spent more time outdoors) compared to the males.
Similarly, to organ tissues, hair samples are the most proper when long-term exposure is suspected [70]. Through their follicles, the hairs are connected to the blood flow, extracting and incorporating not only the nutrients necessary for growth but other elements as well. Because keratin has a good metal-binding affinity, these elements become ‘trapped’ in the cortex and cuticle of the hairs [71], allowing for their testing. As opposed to blood, which is primarily a transport medium and deposits the absorbed substances readily (leading to content fluctuations), hair is a more stable medium for the identification of deposit substances such as minerals and metals [72]. By these characteristics, the hair samples reach a higher similarity relevance as the internal organs for testing chronic exposure to heavy metals irrespective of the exposure source and access ways inside the organism (whether these are air, food, or waterborne pollutants, or if they cross the skin barrier directly from the outside environment).
2.2. Pets as Sentinels for Heavy Metals in Water and Food
Food and waterborne heavy metal intoxications have been documented several times in the scientific literature. The pollution of surface waters with heavy metals is a concerning situation that needs special corrective measures or simply limits on the drinkability of that water source. One such situation exists in Argentina, where many regions are known as chronic regional endemic hydro arsenic zones due to the arsenic contamination of the water. A special contribution to the early detection of chronic waterborne arsenic exposure is canine hair, according to Vázquez et al. [62], with higher values (thus easier to test) than in human hair or the water itself (evidently).
Most of the pollutants in water and food come from the environment, but they significantly increase the health hazards to human and animal populations. The inhabitants of a polluted area (people and pets) will not only be exposed to the direct effect of the pollutant in their environment (from air, soil, contact surfaces) but will receive additional pollutant doses while they eat or drink. In some cases, plant- and animal-origin food is even more dangerous because of the accumulation of pollutants in these. For terrestrial or marine environmental contaminants, such as mercury accumulated in dietary products, sled dogs (Canis lupus familiaris) in the Arctic regions are sentinels for the local communities regarding the possible risks associated with the food they consume. The better they fit this role as mercury absorption, accumulation, and excretion in dogs are similar to humans [73][74][75]. Even the blood-to-hair mercury ratio in dogs (200) is similar to that described in humans [64]. Because there is a highly significant positive correlation between the total mercury in canine blood and hair, dogs can be reliable sentinels of mercury exposure regardless of the sampling method used for quantifying [64][68].
Cats suffer pathological changes from oral methylmercury poisoning similar to those produced in humans suffering from the disease called Minamata disease, discovered in 1959, but whose initial results were never published [76]. The experiment, later repeated by Eto et al. [77], involved sprinkling the cats’ food with liquid mercury, which led to observations related to the accumulation of the metalloid in the cerebrum, cerebellum, liver, and kidney. In both humans and cats, mercury poisoning has the same symptoms, the incoordination known as “dancing cats of Minamata” being an early warning sign of intoxication [78].
Most of the time, oral poisoning with mercury occurs as a result of mercury-polluted pet food. In feline companions, mercury accumulation is influenced by age and sex. In geriatric males, the accumulation begins earlier compared to females and has slightly higher values [67]. Similar to dogs, it was proven that there is a strong correlation between the mercury content in the cats’ hair and that found in their liver and kidneys after the ingestion of mercury-polluted foods. Hence, the conclusion drawn by Skibniewska and Skibniewski [65] is that feline hair can provide an indication of mercury exposure, and its concentration is correlated with the existing loads in other vital organs (liver: 0.030 ± 0.031, kidney: 0.026 ± 0.025 mg⋅kg−1). Wild cat hair lends itself equally well to environmental biomonitoring, according to Behrooz et al. [66].
The first alarm signal about the potential danger that threatens the integrity of human health is the accumulation of mercury values five times higher in the body of pets compared to that in the human body [79].
This entry is adapted from the peer-reviewed paper 10.3390/ani13182923
References
- Sandhu, S.S.; de Serres, F.J. In situ evaluation of biological hazards of environmental pollutants. Mutat. Res. Environ. Mutagen. Relat. Subj. 1989, 216, 341–352.
- Natterson-Horowitz, B.; Bowers, K. Zoobiquity: What Animals Can Teach Us about Health and the Science of Healing; Knopf Doubleday Publishing: New York, NY, USA, 2012; 320p, ISBN 0307593487.
- Beck, A.C.; Lash, E.M.; Hack, J.B. Environmental toxic exposures using companion animals as an indicator of human toxicity: A case report and discussion. J. Emerg. Med. 2020, 59, e1–e7.
- Basu, N.; Scheuhammer, A.M.; Bursian, S.J.; Elliott, J.; Rouvinen-Watt, K.; Chan, H.M. Mink as a sentinel species in environmental health. Environ. Res. 2007, 103, 130–144.
- O’Brien, D.J.; Kaneene, J.B.; Poppenga, R.H. The use of mammals as sentinels for human exposure to toxic contaminants in the environment. Environ. Health Perspect. 1993, 99, 351–368.
- Lazarus, M.; Sekovanić, A.; Orct, T.; Reljić, S.; Kusak, J.; Jurasović, J.; Huber, D. Apex predatory mammals as bioindicator species in environmental monitoring of elements in Dinaric Alps (Croatia). Environ. Sci. Pollut. Res. Int. 2017, 24, 23977–23991.
- Schmidt, P.L. Companion animals as sentinels for public health. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 241–250.
- Pocar, P.; Grieco, V.; Aidos, L.; Borromeo, V. Endocrine-disrupting chemicals and their effects in pet dogs and cats: An Overview. Animals 2023, 13, 378.
- Markert, B.A.; Breure, A.M.; Zechmeister, H.G. Definitions, strategies and principles for bioindication/biomonitoring of the environment. In Trace Metals and Other Contaminants in the Environment; Markert, B.A., Breure, A.M., Zechmeister, H.G., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2003; Volume 6, Chapter 1; pp. 3–39. Available online: https://api.semanticscholar.org/CorpusID:129885601 (accessed on 8 February 2023).
- National Research Council of the United States. Concepts and definitions. In Animals as Sentinels of Environmental Health Hazards; Committee on Animals as Monitors of Environmental Hazards; National Academies Press: Cambridge, MA, USA, 1991; Chapter 2. Available online: https://www.ncbi.nlm.nih.gov/books/NBK234943/ (accessed on 8 February 2023).
- Bukowski, J.A.; Wartenberg, D. An alternative approach for investigating the carcinogenicity of indoor air pollution: Pets as sentinels of environmental cancer risk. Environ. Health Perspect. 1997, 105, 1312–1319.
- Backer, L.C.; Grindem, C.B.; Corbett, W.T.; Cullins, L.; Hunter, J.L. Pet dogs as sentinels for environmental contamination. Sci. Total Environ. 2001, 274, 161–169.
- Moberg, H.L.; Gramer, I.; Schofield, I.; Blackwood, L.; Killick, D.; Priestnall, S.L.; Guillén, A. Clinical presentation, treatment and outcome of canine malignant mesothelioma: A retrospective study of 34 cases. Vet. Comp. Oncol. 2022, 20, 304–312.
- Mesothelioma in Dogs. Available online: https://wagwalking.com/condition/mesothelioma#symptoms (accessed on 12 January 2023).
- Nabeta, R.; Nakagawa, Y.; Chiba, S.; Xiantao, H.; Usui, T.; Suzuki, K.; Furuya, T.; Fukushima, R.; Uchide, T. Pericardial mesothelioma in a dog: The feasibility of ultrasonography in monitoring tumor progression. Front. Vet. Sci. 2019, 6, 121.
- D’Angelo, A.R.; Francesco, G.; Di Quaglione, G.R.; Marruchella, G. Sclerosing peritoneal mesothelioma in a dog: Histopathological, histochemical, and immunohistochemical investigations. Vet. Ital. 2014, 50, 301–305.
- Mott, F.E. Mesothelioma: A review. Ochsner J. 2012, 12, 70–79.
- Rossini, M.; Rizzo, P.; Bononi, I.; Clementz, A.; Ferrari, R.; Martini, F.; Tognon, M.G. New perspectives on diagnosis and therapy of malignant pleural mesothelioma. Fron.Oncol. 2018, 8, 91.
- Lu, J.; Liu, K.; Qi, M.; Geng, H.; Hao, J.; Wang, R.; Zhao, X.; Liu, Y.; Liu, J. Effects of Cr (VI) exposure on electrocardiogram, myocardial enzyme parameters, inflammatory factors, oxidative kinase, and ATPase of the heart in Chinese rural dogs. Environ. Sci. Pollut. Res. Int. 2019, 26, 30444–30451.
- Agency for Toxic Substances and Disease Registry. Case Studies in Environmental Medicine. Chromium Toxicity, 2008, Course WB1466. Available online: https://www.atsdr.cdc.gov/csem/chromium/docs/chromium.pdf (accessed on 19 August 2023).
- Evinger, J.V.; Blakemore, J.C. Dermatitis in a dog associated with exposure to an arsenic compound. J. Am. Vet. Med. Assoc. 1984, 184, 1281–1282.
- Bruere, S.N. Arsenical poisoning in farm dogs. N. Z. Vet. J. 1980, 28, 220.
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human health effects from chronic arsenic poisoning—A review. J. Environ. Sci. Health A. 2006, 41, 2399–2428.
- Singh, N.; Kumar, D.; Sahu, A.P. Arsenic in the environment: Effects on human health and possible prevention. J. Environ. Biol. 2007, 28, 359–365.
- Cohen, S.M.; Ohnishi, T.; Arnold, L.L.; Le, X.C. Arsenic-induced bladder cancer in an animal model. Toxicol. Appl. Pharmacol. 2007, 222, 258–263.
- Dixit, V.P.; Lohiya, N.K.; Agrawal, M. Effect of cadmium chloride on testis and epididymides of dog. A biochemical study. Acta Biol. Acad. Sci. Hung. 1975, 26, 97–103.
- Beynen, A.C. Cadmium in Petfood. 2020. Available online: https://www.researchgate.net/publication/345892225_Beynen_AC_2020_Cadmium_in_petfood (accessed on 20 April 2023).
- Nordberg, G.F.; Nogawa, K.; Nordberg, M. Cadmium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 2, pp. 667–716.
- Oldereid, N.B.; Thomassen, Y.; Attramadal, A.; Olaisen, B.; Purvis, K. Concentrations of lead, cadmium and zinc in the tissues of reproductive organs of men. Reproduction 1993, 99, 421–425.
- Alborough, R.; Grau-Roma, L.; de Brot, S.; Hantke, G.; Vazquez, S.; Gardner, D.S. Renal accumulation of prooxidant mineral elements and CKD in domestic cats. Sci. Rep. 2020, 10, 3160.
- Kim, H.T.; Loftus, J.P.; Mann, S.; Wakshlag, J.J. Evaluation of arsenic, cadmium, lead and mercury contamination in over-the-counter available dry dog foods with different animal ingredients (red meat, poultry, and fish). Front. Vet. Sci. 2018, 5, 264.
- Patočka, J.; Černý, K. Inorganic lead toxicology. Acta Medica (Hradec Kralove) 2003, 46, 65–72.
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64.
- Rana, M.N.; Tangpong, J.; Rahman, M.M. Toxicodynamics of lead, cadmium, mercury and arsenic-induced kidney toxicity and treatment strategy: A mini review. Toxicol. Rep. 2018, 5, 704–713.
- Chang, L.W.; Yamaguchi, S.; Dudley, A.W. Neurological changes in cats following long-term diet of mercury contaminated tuna. Acta Neuropathol. 1974, 27, 171–176.
- Charbonneau, S.M.; Munro, I.C.; Nera, E.A.; Armstrong, F.A.J.; Willes, R.F.; Bryce, W.F.; Nelson, R.F. Chronic toxicity of methylmercury in the adult cat. Interim Report. Toxicology 1976, 5, 337–349.
- Jomova, K.; Valko, M. Mercury toxicity. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1367–1372.
- National Research Council (US), Committee on Animals as Monitors of Environmental Hazards. Animals as Sentinels of Environmental Health Hazards; National Academies Press (US): Washington, DC, USA, 1991. Available online: https://www.ncbi.nlm.nih.gov/books/NBK234946/ (accessed on 19 December 2022).
- Harbison, M.L.; Godleski, J.J. Malignant mesothelioma in urban dogs. Vet. Pathol. 1983, 20, 531–540.
- Otto, C.M.; Hare, E.; Buchweitz, J.P.; Kelsey, K.M.; Fitzgerald, S.D. Fifteen-year surveillance of pathological findings associated with death or euthanasia in search-and-rescue dogs deployed to the September 11, 2001, terrorist attack sites. J. Am. Vet. Med. Assoc. 2020, 257, 734–743.
- Heavy Metal Toxicity in Pets. Available online: https://www.authenticapets.com/en/blog/heavy-metal-toxicity-in-pets (accessed on 10 April 2023).
- Chen, X.; Cao, S.; Wen, D.; Geng, Y.; Duan, X. Sentinel animals for monitoring the environmental lead exposure: Combination of traditional review and visualization analysis. Environ. Geochem. Health 2023, 45, 561–584.
- Park, S.H.; Lee, M.H.; Kim, S.K. Studies on the concentrations of Cd, Pb, Hg and Cr in dog serum in Korea. Asian-Aust. J. Anim. Sci. 2005, 18, 1623–1627.
- Toyomaki, H.; Yabe, J.; Nakayama, S.M.M.; Yohannes, Y.B.; Muzandu, K.; Liazambi, A.; Ikenaka, Y.; Kuritan, T.; Nakagawa, M.; Ishizuka, M. Factors associated with lead (Pb) exposure on dogs around a Pb mining area, Kabwe, Zambia. Chemosphere 2020, 247, 125884.
- Serpe, F.P.; Russo, R.; De Luna, R.; Florio, S.; Esposito, M.; Severino, L. Heavy metal levels in dog liver and kidney in naples (Campania, Italy). In Trends in Veterinary Sciences; Boiti, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 115–117.
- Esposito, M.; De Roma, A.; Maglio, P.; Sansone, D.; Picazio, G.; Bianco, R.; De Martinis, C.; Rosato, G.; Baldi, L.; Gallo, P. Heavy metals in organs of stray dogs and cats from the city of Naples and its surroundings (Southern Italy). Environ. Sci. Pollut. Res. Int. 2019, 26, 3473–3478.
- Forte, G.; Ariu, F.; Bocca, B.; Solinas, G.; Leoni, G.G.; Podda, A.; Madeddu, R.; Bogliolo, L. Heavy metal(loid) accumulation in the ovarian tissue of free-ranging queens and bitches inhabiting highly polluted urban environments. Animals 2023, 13, 650.
- Lanocha, N.; Kalisinska, E.; Kosik-Bogacka, D.I.; Budis, H.; Sokolowski, S.; Bohatyrewicz, A. Comparison of metal concentrations in bones of long-living mammals. Biol. Trace Elem. Res. 2013, 152, 195–203.
- Tomza-Marciniak, A.; Pilarczyk, B.; Bąkowska, M.; Ligocki, M.; Gaik, M. Lead, Cadmium and Other Metals in Serum of Pet Dogs from an Urban Area of NW Poland. Biol. Trace Elem. Res. 2012, 149, 345–351.
- Cedeño, Y.; Miranda, M.; Orjales, I.; Herrero-Latorre, C.; Suárez, M.; Luna, D.; López-Alonso, M. Serum concentrations of essential trace and toxic elements in healthy and disease-affected dogs. Animals 2020, 10, 1052.
- Brownie, C.F.; Brownie, C.; Lopez, V.; Cadogan, P. Dogs and goats as sentinels for environmental lead burden in Caribbean basin islands: Jamaica West Indies. West Indian Vet. J. 2009, 9, 17–20.
- Ward, N.I.; Brooks, R.R.; Roberts, E. Lead levels in whole blood of New Zealand domestic animals. Bull. Environ. Contam. Toxicol. 1977, 18, 595–601.
- Luz, A.L.; Wu, X.; Tokar, E.J. Toxicology of inorganic carcinogens. In Advances in Molecular Toxicology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 12, pp. 1–46.
- Thomas, C.W.; Rising, J.L.; Moore, J.K. Blood lead concentrations of children and dogs from 83 Illinois families. J. Am. Vet. Med. Assoc. 1976, 169, 1237–1240.
- Bischoff, K.; Priest, H.; Mount-Long, A. Animals as sentinels for human lead exposure: A case report. J. Med. Toxicol. 2010, 6, 185–189.
- Berny, P.J.; Côté, L.M.; Buck, W.B. Can household pets be used as reliable monitors of lead exposure to humans? Sci. Total Environ. 1995, 172, 163–173.
- Berny, P.J.; Côté, L.M.; Buck, W.B. Relationship between soil lead, dust lead, and blood lead concentrations in pets and their owners: Evaluation of soil lead threshold values. Environ. Res. 1994, 67, 84–97.
- Pastorinho, M.R.; Sousa, A.C.A. Pets as sentinels of human exposure to neurotoxic metals. In Pets as Sentinels, Forecasters and Promoters of Human Health; Springer: Berlin/Heidelberg, Germany, 2020; pp. 83–106.
- Petrov, E.A.; Nikolovski, G.; Ulčar, I.; Enimiteva, V. Examination of the content of heavy metals using hair samples in dogs of urban areas of Macedonia. Vet. World 2011, 4, 368–370.
- Nikolovski, G.; Atanaskova, E. Use of canine hair samples as indicators of lead and cadmium pollution in the Republic of Macedonia. Bulg. J. Vet. Med. 2011, 14, 57–61.
- Jafari, S. Use of Pets as Indicators of Heavy Metal Exposure Across Sydney. Master’s Thesis, Macquarie University, Sydney, Australia, 2016.
- Vázquez, C.; Rodríguez Castro, M.C.; Palacios, O.; Boeykens, S.P. Risk analysis of acute and chronic exposure to arsenic of the inhabitants in a district of Buenos Aires, Argentina. J. Sustain. Dev. Energy Water Environ. Syst. 2016, 4, 234–241.
- Rodriguez Castro, M.C.; Andreano, V.; Custo, G.; Vázquez, C. Potentialities of total reflection X-ray fluorescence spectrometry in environmental contamination: Hair of owned dogs as sentinel of arsenic exposure. Microchem. J. 2013, 110, 402–406.
- Sousa, A.C.A.; de Sá Teixeira, I.S.; Marques, B.; Vilhena, H.; Vieira, L.; Soares, A.M.V.M.; Nogueira, A.J.A.; Lillebø, A.I. Mercury, pets’ and hair: Baseline survey of a priority environmental pollutant using a noninvasive matrix in man’s best friend. Ecotoxicology 2013, 22, 1435–1442.
- Skibniewska, E.M.; Skibniewski, M. Mercury contents in the liver, kidneys and hair of domestic cats from the Warsaw Metropolitan Area. Appl. Sci. 2023, 13, 269.
- Behrooz, D.; Poma, R.G. Evaluation of mercury contamination in Iranian wild cats through hair analysis. Biol. Trace Elem. Res. 2021, 199, 166–172.
- Sakai, T.; Ito, M.; Aoki, H.; Aimi, K.; Nitaya, R. Hair mercury concentrations in cats and dogs in Central Japan. Br. Vet. J. 1995, 151, 215–219.
- Dunlap, K.L.; Reynolds, A.J.; Bowers, P.M.; Duffy, L.K. Hair analysis in sled dogs (Canis lupus familiaris) illustrates a linkage of mercury exposure along the Yukon River with human subsistence food systems. Sci. Total Environ. 2007, 385, 80–85.
- Aeluro, S.; Kavanagh, T.J. Domestic cats as environmental lead sentinels in low-income populations: A One Health pilot study sampling the fur of animals presented to a high-volume spay/neuter clinic. Environ. Sci. Pollut. Res. Int. 2021, 28, 57925–57938.
- Rosendahl, S.; Anturaniemi, J.; Vuori, K.A.; Moore, R.; Hemida, M.; Hielm-Björkman, A. Diet and dog characteristics affect major and trace elements in hair and blood of healthy dogs. Vet. Res. Commun. 2022, 46, 261–275.
- Gil-Jiménez, E.; Mateo, R.; de Lucas, M.; Ferrer, M. Feathers and hair as tools for non-destructive pollution exposure assessment in a mining site of the Iberian Pyrite Belt. Environ. Pollut. 2020, 263, 114523.
- Chojnacka, K.; Zielińska, A.; Michalak, I.; Górecki, H. The effect of dietary habits on mineral composition of human scalp hair. Environ. Toxicol. Pharmacol. 2010, 30, 188–194.
- Amadi, C.N.; Frazzoli, C.; Orisakwe, O.E. Sentinel species for biomonitoring and biosurveillance of environmental heavy metals in Nigeria. J. Environ. Sci. Health C 2020, 38, 21–60.
- Harley, J.R.; Bammler, T.K.; Farin, F.M.; Beyer, R.P.; Kavanagh, T.J.; Dunlap, K.L.; O’Hara, T.M. Using domestic and free-ranging Arctic canid models for environmental molecular toxicology research. Environ. Sci. Technol. 2016, 50, 1990–1999.
- Klejka, J. Using hair as an Indicator of Mercury Exposure in Sled Dogs. 2012. Available online: https://scholarworks.alaska.edu/bitstream/handle/11122/1529/KlejkaJ.pdf?sequence=1&isAllowed=y (accessed on 14 April 2023).
- Rabinowitz, P.; Scotch, M.; Conti, L. Human and animal sentinels for shared health risks. Vet. Ital. 2009, 45, 23–24.
- Eto, K.; Yasutake, A.; Nakano, A.; Akagi, H.; Tokunaga, H.; Kojima, T. Reappraisal of the historic 1959 cat experiment in Minamata by the Chisso factory. Tohoku J. Exp. Med. 2001, 194, 197–203.
- Reif, J.S. Animal sentinels for environmental and public health. Public Health Rep. 2011, 126, 50–57.
- Polluted Pets. High Levels of Toxic Industrial Chemicals Contaminate Cats and Dogs. 2008. Available online: https://www.ewg.org/research/polluted-pets (accessed on 22 January 2023).
This entry is offline, you can click here to edit this entry!
