Gestational diabetes mellitus (GDM) is one of the most common metabolic diseases encountered in obstetrics, and it is often associated with an increased risk of adverse pregnancy outcomes. GDM affects about 9–25% of pregnancies worldwide. The frequency of GDM increases with the age of a pregnant woman.
- gestational diabetes mellitus
- biomolecules
- predictor
1. Introduction
Gestational diabetes mellitus (GDM) is one of the most common metabolic diseases encountered in obstetrics, and it is often associated with an increased risk of adverse pregnancy outcomes [1,2]. GDM affects about 9–25% of pregnancies worldwide [3]. The frequency of GDM increases with the age of a pregnant woman. Glucosuria, excessive weight gain, polyhydramnios, and fetal macrosomia during pregnancy are indications for further diagnosis of carbohydrate metabolism disorders [4]. The algorithm for detecting GDM includes performing an oral glucose tolerance test between 24 and 28 weeks of gestation or as soon as possible if the pregnant woman is in a risk group. In clinical practice, a pregnant woman’s fasting blood glucose level is checked at her first visit [5,6]. Patients with a history of overweight or obesity, elevated blood glucose levels or impaired glucose tolerance prior to pregnancy, age over 35 years, family history of diabetes, previously giving birth to a baby with a birth weight larger than 4000 g, obstetrical failures in previous pregnancies (i.e., miscarriages, malformations, intrauterine deaths), with glucose in the urine, or increased amniotic fluid are significantly more likely to develop GDM and diabetes mellitus type 2 (T2DM) in the future [7]. Undiagnosed GDM carries the risks of obstetric complications such as: polyhydramnios, preterm delivery, edema, urinary tract infections, pyelonephritis, fetal macrosomia, or preeclampsia [8]. Thus, GDM therapy should include monitoring of several components, i.e., blood glucose levels, the presence of ketones in urine, weight gain, fetal biometry, and physical activity.
Pathogenesis of GDM is complex and, inter alia, it involves impairment of the action and secretion of insulin. Insulin resistance during pregnancy develops gradually, and finally, in consequence, it leads to hyperglycaemia [9,10]. Glucose, which passes through the placenta, is the main source of energy for the developing fetus. Therefore, the response to the elevated glucose level is increased insulin production by the fetus, which in turn causes muscle tissue hypertrophy, including the heart muscle, fatty tissue, and the liver [11,12]. In consequence, excessive fetal growth (macrosomia) is observed. Moreover, increased levels of hormones in a pregnant woman, i.e., estrogen, progesterone, and placental lactogen, as well as other hormones, increase insulin resistance even further. Surely, excessive body fat, low physical activity, hypertension, age, and family history of diabetes may be conductive to intensify this process [13].
Pregestational obesity and excessive weight gain during pregnancy seem to be the main causes of GDM [15,19]. The adipose tissue is able to secrete hormones, i.e., adipokines, which may contribute to insulin resistance. A “satiety hormone” called leptin impairs insulin-dependent glucose transport to adipocytes. It mediates the increased protein synthesis and may directly impair glucose secretion by the beta cells of the pancreas. During pregnancy, leptin levels increase because leptin is produced by the placenta as well [20,21,22,23,24,25]. The highest increase in leptin concentration is observed in the second trimester of pregnancy, while its concentration decreases rapidly just after delivery. Leptin can act as a metabolic switch connecting the nutritional status of the body to the high energy-consuming processes [26]. Obese pregnant women have significantly elevated plasma leptin concentrations throughout pregnancy compared with non-obese pregnant women throughout pregnancy. The high plasma leptin levels may be potentiated by leptin resistance at the central level [27]. The effects of placental leptin on the mother may contribute to the endocrine-mediated alterations in the energy balance, such as mobilization of maternal fat, which could further aggravate the insulin resistance associated with pregnancy and the onset of GDM [28].
The placenta is the organ linking the mother to the fetus [29]. As an endocrine organ, it secretes many cytokines and adipokines, including adiponectin, which mediate fetal development and maternal metabolism [18,30]. In the presence of placental hormones, the body cells become insensitive to insulin, which causes an increase in blood glucose levels. As the baby grows in size, the placenta produces more and more hormones, which results in hyperglycaemia.
2. Biomolecules
Yang et al. conducted a study in which they compared chemerin levels in women with and without GDM during the first and third trimesters [69]. They noted a significant increase in chemerin in women with GDM in the third trimester compared to healthy women. Interestingly, chemerin levels during the first trimester were higher in women without GDM. This could explain why, in some studies, chemerin levels were lower in women with GDM compared to healthy ones.
The use of chemerin levels in assessing the effectiveness of treatment for patients with GDM was also considered. The purpose of this was to predict whether patients with GDM would need pharmacological treatment or whether dietary therapy would be sufficient. However, there was no correlation between chemerin levels and poor glucose monitoring in these patients [56].
It is suggested that its disfunction disrupts glucose homeostasis, induces the development of insulin resistance, and thus contributes to the development of diabetes [72]. Omentin-1 is thought to be able to increase glucose uptake in adipocytes and has insulin sensitivity-enhancing abilities. It has been noted that its levels are reduced in patients with obesity and diabetes [73]. Interestingly, according to some studies, it is possible to regulate omentin-1 levels in these patients. It has been observed that weight loss can increase levels of this adipokine in obese patients with T2DM [74]. It has also been noted that omentin-1 levels are increased in pre-diabetic states, suggesting that this adipokine may increase insulin sensitivity, and its secretion is increased as a defense mechanism in the early stages of glucose intolerance [75].
Pan et al. conducted a detailed meta-analysis in which they examined 42 studies on the effect of omentin-1 levels on the development of different types of diabetes [65]. In 32 of these studies, the levels of this adipokine were significantly reduced in patients with T2DM compared to healthy subjects. Moreover, 7 of the studies comparing omentin-1 levels in patients with GDM and healthy controls suggested similar findings. Omentin-1 concentrations were significantly lower in women with GDM. However, these studies showed remarkably high heterogeneity, indicating that some non-described factors might have been responsible for those outcomes.
3. Conclusions
Most of the original scientific studies presented in this review are retrospective, which means that they were carried out in pregnant women already diagnosed with GDM. Hypotheses and pathways of these 13 less known biomolecules in the pathogenesis of GDM are presented in Figure 1 .
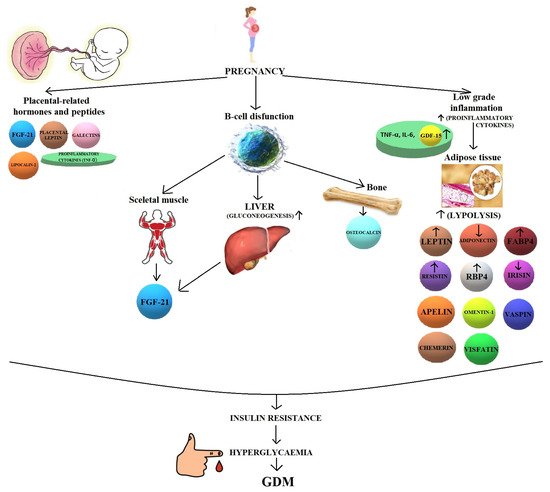
Moreover, 7 of them present controversial data concerning the serum concentrations in women with GDM compared to healthy pregnant women ( Figure 2 ).
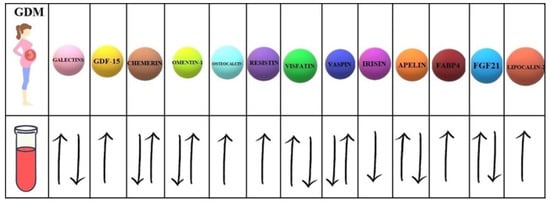
Considering the non-invasive nature of collecting saliva or urine samples, it seems that such biomaterials would be the most optimal factors in predicting any disease. Out of many biomolecules circulating in the blood, chemerin and resistin contained in the saliva are worth paying attention to since their levels are reported to be significantly higher in women with GDM in comparison to healthy women. The above-described observations may be suggestive of the validity of performing non-invasive tests, the use of which could detect the risk of developing GDM.
Hopefully, future clinical trials will shed more light on the validity of these hypotheses and, more importantly, determine which biomolecule has the most potential to predict GDM pathogenesis.
This entry is adapted from the peer-reviewed paper 10.3390/ijms222111578
