The main function of interstitial cells of Cajal (ICCs) is to regulate gastrointestinal peristalsis by acting as a “pacemaker” cell by generating spontaneous slow electrical waves. In 2005, electron microscopy revealed a cell type similar to ICCs (ICC-like) outside the gastrointestinal tract, with contractile activity and c-Kit+ immunohistochemistry shared with ICCs. Among the locations where ICC-like cells have been observed, it is in the uterus where they have a significant functional and pathophysiological role. These cells are involved in obstetric phenomena of contractile action, such as ascending sperm transport, embryo implantation, pregnancy, delivery, and the expulsion of menstrual debris.
1. Introduction
1.1. Ultrastructural and Immunohistochemical Characteristics
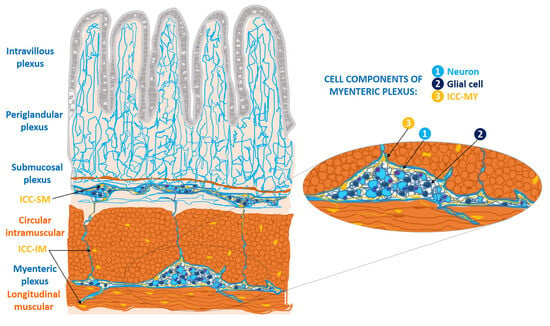
ICCs of the wall of the gastrointestinal tract form cell networks with connections to the enteric nerve plexuses. They are classified into three major subtypes: myenteric ICCs (ICC-MY), intramuscular ICCs (ICC-IM), and submucosal ICCs (ICC-SM). ICC-my are located at the level of the myenteric (Auerbach’s) plexus, between the longitudinal and circular muscle layers; ICC-IM are located in the thickness of both muscular layers; and ICC-SM constitute the cellular network located in the submucosal (Meissner) plexus [
5] (
Figure 1).
Figure 1. Location of cellular subtypes of interstitial cells of Cajal (ICCs) in the wall of the digestive tract (ICC-MY, ICC-IM, ICC-SM), based on a schematic drawing made by Santiago Ramón y Cajal [
1]. ICC-MY: ICCs of the myenteric plexus, between the longitudinal muscle fibers and the circular muscle fibers; ICC-IM: ICC intramuscular at the level of both muscular layers; ICC-SM: ICCs of the submucosal plexus, and fibers of submucosal plexus, connecting with the myenteric plexus. The periglandular plexus in the Lieberkühn’s glands; and the intravillous plexus in the intestinal villi. In detail: the components of myenteric plexus, in which there is a ganglion constituted by neurons, glial cells, and the ICC-MY [
5].
EM visualization of ICC subtypes at the different locations shows that they share similar ultrastructural characteristics. They have spindle-shaped or oval cell bodies with long cytoplasmic extensions of 100 μm or more, which may branch into secondary and tertiary extensions [
6]. Their plasma membranes have surface caveolae and a discontinuous basal lamina. The cytoplasmic organelles include abundant mitochondria, smooth endoplasmic reticulum cisternae, microtubules, fine and intermediate cytoskeletal filaments, free ribosomes, a discrete Golgi apparatus, rough endoplasmic reticulum and lysosomes. Also, these cells present gap-junction type membrane proteins that allow them to establish intercellular connections with neighboring smooth muscle cells [
6].
Beyond their recognized ultrastructural features, ICCs present a series of defining staining and immunohistochemical characteristics [
5]. They show positive staining for methylene blue and osmic acid/zinc iodine and, in immunohistochemical assessment, ICCs stain positively for the membrane receptor c-Kit [
5]. This is a tyrosine kinase–type transmembrane receptor that binds to stem-cell growth factor (SCF), thus, regulating cell proliferation and division. It is encoded by the
KIT proto-oncogene and plays a fundamental role in the transduction of growth and repair signals [
7]. Antibodies to c-Kit and its epitope CD117 are used to immunolabel ICCs, which are thus considered CD117/c-Kit
+ cells [
5].
1.2. Functions
Current knowledge relates the three main functions of these cells to their gastrointestinal location: pacemaking for the peristalsis of the digestive tract, enteric neurotransmission and mechanoreception, and an important role in regulating the muscle tone of the wall [
5]. First, ICCs’ pacemaker properties refer to their involvement in the regulation of gastrointestinal peristaltic activity, as they have the capacity to generate spontaneous, slow electrical waves in the gastrointestinal tract [
9,
10]. Those slow electrical waves, whose fine mechanism is unknown, are associated with cytoplasmic oscillations of intracellular Ca
2+ ions, possibly by releasing Ca
2+ from intracellular stores.
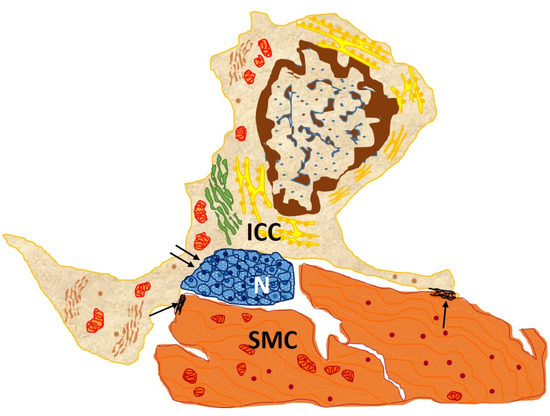
Second, ICCs participate in enteric neurotransmission by receiving excitatory and inhibitory neuronal stimuli from the enteric nervous system and transmitting them to neighboring smooth muscle cells. They thus stand in the middle of a functional neuromuscular network, establishing synapses with neurons and communicating with smooth muscle cells through gap junctions (Figure 2).
Figure 2. Drawn from EM image from the circular muscle layer of rabbit colon showing an interstitial cell of Cajal (ICC), with an elongated body and three processes located in the connective interstitium, which contacts, through gap junction, between both its body and processes and two SMC (arrows) and nerve endings (double arrows). This cell owns the typical features of an intramuscular ICC: conspicuous Golgi apparatus and several rough endoplasmic reticulum cisternae; basal lamina is thin and discontinuous [
13].
Finally, ICCs also function as mechanoreceptors. ICCs collect continuous mechanical signals, originating in the distensibility of the gastrointestinal walls, which occurs during food propulsion, digestion, and waste elimination.
1.3. Disfunctions
Taking into account these three primary functions described above, it is understandable that changes in ICC numbers are associated with gastrointestinal motility disorders [
14]. Several pathologies of childhood and old age have been associated with variations in the number of ICCs, as demonstrated by c-Kit immunolabeling. This is the case in pediatric conditions such as Hirschprung’s disease, the most prevalent congenital gastrointestinal motility disorder, a functional intestinal obstruction resulting from a defect in the intrinsic innervation of the intestine. Since ICCs have a central role in gastrointestinal peristalsis and enteric neurotransmission, their quantitative decrease may explain the motility dysfunction that occurs in this pediatric disease [
15]. At older ages, a decrease in ICC numbers has been related to disorders such as acquired hypoganglionosis, diabetic or idiopathic gastroparesis, intestinal pseudo-obstruction, and chronic constipation [
14,
16]. Finally, and independently of age, megacolon associated with Chagas disease could also be explained by a lower density of ICCs. This is a “megasyndrome” defined as a chronic dilatation of the colonic segment, associated with infection by the
Trypanosoma cruzi parasite and the destruction of the enteric nervous system [
17].
The pathophysiological correlation of ICCs with disease has been studied in gastrointestinal stromal tumors (GISTs). In GISTs, mutation of the
c-kit gene leads to SCF-independent activity, triggering cell division and genomic instability that result in neoplasia. ICC-derived GISTs can be identified by their immunohistochemical positivity for vimentin, CD34, and c-Kit [
18,
19]. These markers have allowed us to understand the pathogenesis of GIST and have made it possible to develop an effective treatment targeting c-Kit.
2. ICC-like Cells Outside the Digestive System
2.1. Ultraestructural Characteristics
Broadly speaking, ICC-like cells share with ICCs some cytoplasmic features and membrane properties. The typical cytoplasmic extensions define the stellate morphology of ICC-like cells. Here there are highlighted some of their morphologic characteristics:
- -
-
They are of great length, tens to hundreds of μm, and considered one of the longest structures in the human body [
27];
- -
-
They vary in number from one to five, there frequently being two or three per cell. Thus, the three-dimensional appearance of ICC-like cells is that of a polyhedron with a variable number of vertices according to the number of extensions;
- -
-
They are thin, <0.2 μm, and moniliform in appearance, with dilations along their length harboring Ca
2+ channels and cytoplasmic organelles [
27].
These specific characteristics of the ICC-like cells’ extensions distinguish them from other cytoplasmic cellular extensions such as dendrites and axons, precluding any type of relationship between ICC-like cells and neurons.
2.2. Immunohistochemical Characteristics
Apart from CD117/c-Kit positivity shared with ICCs of the gastrointestinal tract, ICC-like cells present in their membrane a series of proteins (CD34, vimentin, platelet-derived growth factor receptors alpha and beta [PDGFR-α and β], caveolin-1, CD44, stem cell antigen-1 [Sca-1], Nanog and octamer-binding protein 4 [Oct-4]) that make their immunohistochemical recognition distinctive.
2.3. Functions of ICC-like Cells
The main recognized function of ICC-like cells is intercellular signaling, taking advantage of their strategic position between blood vessels, nerves, and other adjacent cells. This paracrine/juxtacrine type of intercellular signaling, mediated by the shedding of microvesicles in the form of exosomes, ectosomes, and multivesicles, occurs in a homocellular and heterocellular manner [
28,
29]. In the case of homocellular communication, it takes place between two extensions of ICC-like cells or between one extension and the body of an ICC-like cell. In heterocellular signaling, ICC-like cells communicate with different cell types, such as fibroblasts, myofibroblasts, pericytes, endothelial cells, neurons, stem cells, macrophages, mast cells, eosinophils, lymphocytes, plasma cells, Schwann cells, cardiomyocytes, and smooth muscle cells [
22].
These heterocellular communications allow ICC-like cells to modulate the immune response, regulate blood flow, and contribute to tissue regeneration and repair, the organization of the extracellular matrix, cell migration, and the maintenance of tissue homeostasis. In addition, by producing vascular endothelial growth factor (VEGF), they play an angiogenic role; moreover, by producing abundant superoxide dismutase (SOD2), they have antioxidant properties. Considering all these functions, the use of ICC-like cells in regenerative medicine as a therapy is a possibility [
22].
3. ICC-like Cells in Female Genital Tract
In the female genital tract, ICC-like cells have been linked to the generation of slow electrical waves at the tubal level that trigger the contractility necessary for egg transport [
33]. Similarly, in the myometrium, electrical waves linked to ICC-like cells have possibly been detected [
30]. However, in this topic there is still a great deal of uncertainty to be resolved. Some hypotheses advocate that it is, above all, in organs with regular contractile activity, such as the pancreas and other exocrine secreting glands, that this pacemaker function of ICC-like cells is active [
2,
3,
29].
3.1. Ultraestructural and Immunohistochemical Features
In the uterus, ICC-like cells are located in the endometrium and myometrium [
21]. The myometrium is composed of three poorly differentiated layers of smooth muscle bundles: the inner longitudinal layer, the vascularized middle circular layer, and the outer longitudinal layer [
34]. It is between the muscle bundles of the different layers that the myometrial ICC-like cells are found [
21]. The endometrium is the innermost layer of the uterus and the one with the greatest number of histological components. It is made up mainly of connective tissue, glands, and stromal cells that, during gestation, are transformed into decidual cells.
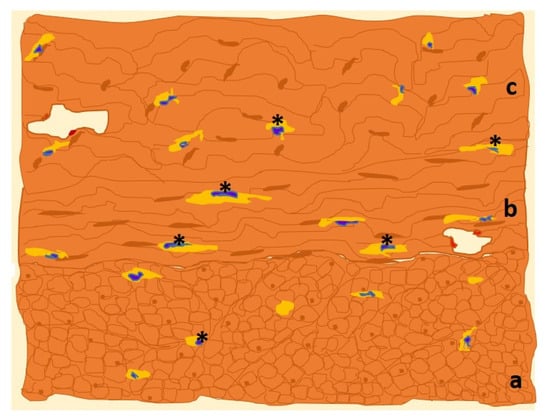
As in other locations, uterine ICC-like cells express plasma membrane CD117/c-Kit protein, which is frequently targeted for immunohistochemical detection (Figure 3).
Figure 3. Drawn of MO image of a human uterine myometrial tissue sample in which immunohistochemistry for c-Kit has been used. The immunolabeled ICCs-like cells/telocytes (*, in yellow) are identified and distributed along the three constituent layers of uterine smooth muscle: outer longitudinal (a), middle circular (b) and inner longitudinal (c) layers [
36].
Other frequent targets for immunolabeling in the uterus are CD34 and PDGFR-α and β proteins. CD34 is preferentially located in the plasma membrane of uterine ICC-like cells’ cytoplasmic extensions, whereas PDGFR-α and β are more prevalent in the cell body.
Uterine ICC-like cells exhibit plasma membrane estrogen receptor alpha (ERα) and progesterone receptor-A (PR-A), which, interestingly, are also found in the uterine tubes [
21]. This common phenotype suggests that uterine ICC-like cells’ activities are modulated by levels of circulating sex hormones, which further suggests an additional cyclic regulatory role in organ response.
3.2. Functions of Uterine ICC-like Cells
The fundamental role of uterine ICC-like cells is the same as that of ICC-like cells in other extragenital locations—to trigger contractile activity [
30].
In utero, variations in myometrial contractility are a part of both normal pregnancy and delivery and their pathological forms of miscarriage and premature delivery. These events occurring in the female genital tract, together with other pathological findings such as leiomyomas and endometriosis, have a certain relationship with uterine ICC-like cells, as detailed below [
21,
37].
3.2.1. ICC-like Cells Implications in the Physiology of Pregnancy and Labor
The myometrial contractile activity associated with uterine ICC-like cell functioning favors the upward transport of sperm prior to fertilization, embryo implantation, variations in uterine capacitance during pregnancy or delivery, and the expulsion of menstrual debris [
37]. This contractility triggered by uterine ICC-like cells is associated not only with intracellular oscillations in Ca
2+ concentrations, but also with their quantitative variations, which are modulated by signaling through their membrane proteins [
21]. These include the immunohistochemical markers connexin 43 and CD117/c-Kit, the hormone receptors ERα and PR-A, and the transmembrane channels Cav3.1, Cav3.2, and SK3 [
28,
29,
30].
In the physiology of gestation and childbirth, some of the hormonal alterations mentioned are related to the activity of uterine ICC-like cells. In fact, the plasma-membrane protein components of uterine ICC-like cells, such as ERα and PR-A receptors and the immunomarker connexin 43, not only increase in expression as gestation progresses, they also play a role in the physiology of uterine contractions of labor [
28,
30].
Labor is a complex physiological process, defined by numerous hormonal interactions that trigger uterine contractions that, ultimately, lead to fetal expulsion. At “term”—considered as the time interval between 37 and 40 weeks plus 6 days of gestation—there is an imbalance between the myorelaxant hormones that favor pregnancy (progesterone, nitric oxide, catecholamines, and relaxin) and those that trigger labor (estrogens, oxytocin, and prostaglandins), in favor of the latter.
ERα and PR-A promote overexpression of T-type Ca
2+ channels in the ICC-like cell membrane, triggering the continued uterine contractions characteristic of term [
27]. The localization of the gap junction protein connexin 43 in uterine ICC-like cells allows the establishment of multiple connections with surrounding myocytes. Together with the involvement of estrogens, oxytocin, and prostaglandins, the result is the development of regular forceful contractions.
It is argued that the peculiar widespread localization and gap-junction nature of connexin 43 at the time of parturition provides the means for triggering a synchronous contraction of all uterine smooth muscle cells, constituting a “functional syncytium” [
30]. However, this view has been challenged and an alternative model of “biphasic” uterine contraction, defined by a fast electrical wave followed by a slower one, has been advocated.
There is, thus, a considerable amount of information favoring the existence of uterine ICC-like cells, with distinct specific localization and distribution patterns of molecules. These favor their involvement in uterine contractions and in local signaling that regulates the frequency of contractions and the surveillance of signals related to labor conditions.
3.2.2. Physiopathological Implications
Due to their distribution, uterine ICC-like cells are implicated in gynecological and obstetric disorders. They are linked to recurrent miscarriages, premature deliveries, abolition of uterine contractions, and implantation failures. Beyond obstetric pathophysiology, associations of these cells with pathological conditions of the non-pregnant uterus such as endometriosis and leiomyoma have also been described [
21,
37].
Gynecological disorders
Uterine fibroma (or leiomyoma), is the most common benign tumor of the female genital tract: worldwide, around 50% of women present it during their fertile life [
8]. Clinically, these tumors manifest as chronic pelvic pain, abundant bleeding, spontaneous abortions, and infertility, all associated with deterioration in the quality of life [
8]. There is an imbalance in the three-dimensional organization of the uterine tissue in favor of an excessive increase in the number of fibroblasts and myocytes [
37].
Leiomyomas are described as steroid-hormone sensitive and even as hormone-dependent [
36]. Estrogen receptors and, to a lesser extent, progesterone receptors, are overexpressed in myocytes [
50], which under estrogen signaling release growth factors such as VEGF or PDGF. The enhanced nervous density in leiomyomas, greater than in healthy myometrium, has been demonstrated by means of positive immunoreactivity for markers of autonomic innervation such as protein gene product 9.5 (PGP 9.5) and inducible nitric oxide synthase (iNOS) [
51].
Leiomyomas are tumors that contain uterine ICC-like cells, but smooth muscle cells and fibroblasts predominate [
37]. The balance between these cell types oscillates according to variation in the dimensions of the tumor, which reflect the cell density. When smooth muscle cells and fibroblasts are disproportionately increased in number, this physiological balance is broken and uterine c-Kit
+ ICC-like cells decrease in number or disappear completely [
52].
ICC-like cells are known to have an intermediary role in tumor innervation, through signaling by peritoneal macrophages to synthesize iNOS, which is used as an immunohistochemical marker for autonomic innervation detection [
53]. The close spatial relationship between uterine ICC-like cells and myometrial autonomic nerve fibers has been demonstrated by double-positive immunostaining for both iNOS and PGP 9.5 as well as CD34 in the same sample [
51].
Finally, uterine ICC-like cells, by sharing intercellular signals with myometrial mast cells and myocytes, can participate in tissue remodeling and growth. This is because myometrial mast cells have surface receptors for SCF, a smooth-muscle-cell factor and modulator of mast cells’ role in tissue remodeling. This contributes to more extensive consequences involving local inflammation and further leiomyoma growth [
37].
Obstetric disorders
Pathological conditions linked to pregnancy and childbirth are influenced by membrane components of the uterine ICC-like cells, together with the intercellular communications that they establish with adjacent cells. That is the case for recurrent abortions, abolition of uterine contractions, premature delivery, and implantation failure [
37].
Recurrent or repeated spontaneous abortions are defined as a succession of three or more consecutive abortions of an embryo or fetus weighing 500 g or less [
39]. These recurrent miscarriages may be due to multiple causes, including connexin-43 protein deficiency. This protein, apart from participating in the contractile activity linked to parturition, also favors the maturation of the decidua [
28,
29], whose cells differentiate from endometrial cells and are eliminated with menstruation.
Preterm deliveries are defined as those occurring before 37 weeks of gestation [
39]. In these cases, it is desirable that myometrial ICC-like cells density decrease to prevent contractions and prematurity [
28].
Embryo implantation failure results from defective intercellular signaling between ICC-like cells and cells of the immune system that is likely to destabilize immunosurveillance of the uterine environment. Miscarriage and endometriosis appear to result from the same defect. These three conditions also appear to derive from peritoneal macrophages’ abnormal production of IL-6, IL-10, IL-1R1, and TNFα [
53].
4. Conclusions
ICCs are c-Kit+ cells located largely in the wall of the gastrointestinal tract where, through an extensive network, they generate slow electrical waves and exert a pacemaker function, regulating gastrointestinal peristalsis. Among the pathologies with which they have been associated, GIST tumors stand out.
On account of their characteristic ultrastructure, with long moniliform cytoplasmic extensions, and a predominance of positive CD34 and c-Kit immunohistochemistry, ICC-like cells are recognized in the uterine tissue, where they mediate immunosurveillance, myometrial regeneration, and contractility.
Uterine ICC-like cells’ contractility is relevant to the physiology of pregnancy and childbirth. Quantitative oscillations of cell density and the ICC-like cell membrane receptors ERα, PR-A, and others, such as connexin 43, further contribute to the contractility of parturition. Finally, uterine ICC-like cells’ intervention or density may contribute to the pathophysiology of leiomyomas, recurrent miscarriages, and preterm deliveries.
This entry is adapted from the peer-reviewed paper 10.3390/cimb45090476