Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Aortic and visceral aneurysms affect large arterial vessels, including the thoracic and abdominal aorta, as well as visceral arterial branches, such as the splenic, hepatic, and mesenteric arteries, respectively.
- cardiothoracic surgery
- vascular surgery
- tissue engineering
- mesenchymal stem
- miRNA
- cell therapy
- vascular biology
1. Definition and Epidemiology of Aneurysms
According to the 2014 European Society of Cardiology and the corresponding guidelines published in 2022 by the American College of Cardiology, a uniform definition for aneurysms does not exist. Descending thoracic aortic (TAA) and abdominal aortic aneurysms (AAA) are defined as any dilatation of at least 1.5 times the initial diameter of the affected vessel. Regarding the ascending aorta, an increase in diameter greater than 4.0 cm is considered a dilatation, while an increase greater than 4.5 cm is defined as an aneurysm [8].
With an estimated incidence of 5.9 per 100,000 person-year, which is constantly increasing [9], a thoracoabdominal aneurysm (TAAA) is defined as an aortic aneurysm that may extend into both the thoracic and the abdominal aorta. TAAAs may be categorized according to the Crawford Classification into five types, which in general, describe aneurysm morphology and aid in patient risk stratification [9,10].
On the other hand, visceral artery aneurysms (VAA), i.e., aneurysms affecting various visceral arteries, including the splenic, the hepatic and the mesenteric arteries, have a population incidence of about 0.01% to 0.1%, and are thus considered relatively rare [11]. Furthermore, within this cohort, splenic artery aneurysms seem to be the most frequent, comprising 60% of the cases [11].
2. Etiology of Aortic and Visceral Aneurysms: Genetic Syndromes vs. Sporadic Disease
Typically, aortic aneurysms occur sporadically. Tobacco abuse, uncontrolled hypertension, older age, and even an untreated chronic aortic dissection are considered the main predisposing factors [9,12]. Atherosclerosis, although more frequently implicated in descending thoracic and abdominal aortic aneurysms, might also be an etiologic factor for TAAAs and VAAs [13,14], while inflammatory disorders such as Takayasu arteritis, infectious aortic disease, or trauma may also be to blame [8]. Moreover, hemodynamic alterations, or even hormonal changes during pregnancy, in the case of splenic artery aneurysms for example, might be predisposing factors as well [11]. Aortic aneurysms have also been associated with various genetic disorders, including but not limited to Marfan Syndrome (MFS), type IV Ehlers-Danlos Syndrome (EDS) (vascular EDS) [15], autosomal dominant polycystic kidney disease (ADPCKD), and Turner Syndrome (XO). Many of these syndromes have been linked with both thoracic and abdominal aortic diseases [16,17,18,19,20,21]. Moreover, vascular Ehlers-Danlos syndrome seems to be responsible for genetic predisposition to VAA occurrence as well [22].
3. Symptomatology of Aortic and Visceral Aneurysms
Though initially asymptomatic, as the aneurysm progresses, pressure on adjacent tissues might cause symptoms in about 57% of patients prior to rupture, including hoarseness and dysphagia due to compression of the left laryngeal nerve or esophagus, respectively, as well as gastrointestinal obstruction due to impingement on the nearby small intestine [23]. TAAAs in particular, may be also the cause of vague and chronic chest or abdominal pain, with signs of erosion typically presenting as hemoptysis or hematemesis, whereas VAAs, though also usually asymptomatic, might present with intraperitoneal hemorrhage and bleeding from the portal venous system [24]. In addition, retrograde expansion of some TAAAs, as well as thoracic aortic aneurysms (TAAs), might lead to dilation of the aortic annulus, causing aortic valve regurgitation and aortic dissection [25].
4. Molecular Pathophysiology of Aortic and Visceral Aneurysms
Aneurysms generally involve derangement of normally occurring molecular processes in the aortic wall. In general, every cell type within this microenvironment may be affected and contribute alone or together with others towards aneurysm formation and progression. Endothelial cells (ECs) may contribute to aneurysmal pathogenesis through derangement in the expression of cell adhesion molecules, or expression of pro-inflammatory cytokines, including TNF-a. These molecules may lead to increased leukocyte adhesion, oxidative stress, and inflammation within the wall [26], causing endothelial injury. This is further supported by relevant studies in animal models of TAA, which have shown that when intercellular EC adhesion molecules, such as tight junctions (TJs) and adherens junctions (AJs), are preserved, no TAA formation is observed [27]. It therefore seems that inflammation indeed plays a role at this stage of pathogenesis. Furthermore, as it is a characteristic finding in AAA, it could be also be implied in TAAA formation [9], while it is implicated in VAAs pathogenesis as well, especially celiac artery and mesenteric artery aneurysms [28,29]. ECs, in this case, may often display a pathological phenotypic change, termed epithelial to mesenchymal transition (EpMT), which includes loss of cellular adhesion characteristics, and acquisition of a mesenchymal phenotype, along with increased protein secretion, tendency for migration, and leukocyte adhesion molecule expression; this further disrupts the endothelial barrier, augmenting circulating leukocyte recruitment, and as a result, inflammation [30,31].
Another important factor is disruption of the normal extracellular matrix ECM turnover; increased matrix metalloproteinase (MMP) activation leads to increased collagen and elastin hydrolysis [32,33], in turn causing unopposed ECM degradation. Two specific metalloproteinases have been found to be implicated; MMP9 has been associated with aneurysmal wall dilatation [34], while MMP2 has been identified in higher quantities in disease-free segments. Furthermore, altered expression of tissue inhibitor of metalloproteinases (TIMPs) [35] has also been linked to disease pathogenesis, as shown in relevant animal models.
Phenotypic derangement of vascular smooth muscle cells (VSMCs) and their subsequent apoptosis [33,36] might also be a contributing factor; though a normal equilibrium between synthetic (sVSMC) and contractile VSMCs (cVSMCs) populations does normally exist, inflammatory conditions in the aortic wall have been shown to favor VSMC phenotype cycling towards a more proliferative state, leading to an alteration in aortic contractile properties. These proliferative VSMCs are also abnormal, since they have been shown to express both contractile and synthetic protein markers, and are more susceptible to apoptosis as well, eventually causing thinning of the tunica media [31]. It thus seems that progressive weakening of the medial layer, which can sometimes be attributed to an alteration in flow and hemodynamic conditions, as is the case with splenic artery aneurysms for example [29,37], is central to the development of both aortic aneurysms and VAAs [38].
A number of intracellular and extracellular signaling pathways have been found to be associated with aneurysm development in the aorta; one is the MAPK/ERK pathway, which, through sequential kinase activation, triggers NF-Kβ expression [39], although impairment of the AKT2 pathway may also contribute to aneurysm formation [40,41]. Disrupted TGF-b signaling may also be implicated in pathogenesis, due to possible alterations in VSMC and EC phenotype, causing these cells to acquire mesenchymal characteristics; eventually, loss of the normal cell phenotype occurs, with disturbance of the endothelial barrier, and eventual leukocyte infiltration within the aortic wall [31]. In addition, TGF-b signaling may affect the relative quantities of extracellular fibrillar proteins and cellular cytoskeleton organization, thus affecting cellular movement. Finally, altered TGFB receptor functions may affect the MAPK pathway as well [42,43].
Shiz Aoki; Katya Shteyn; and Ryan Marien; Figure 1: Physiology of the aortic wall and pathophysiology of an aortic aneurysm—created with BioRender.com. Available online: https://www.biorender.com/about (accessed on 18 July 2023).
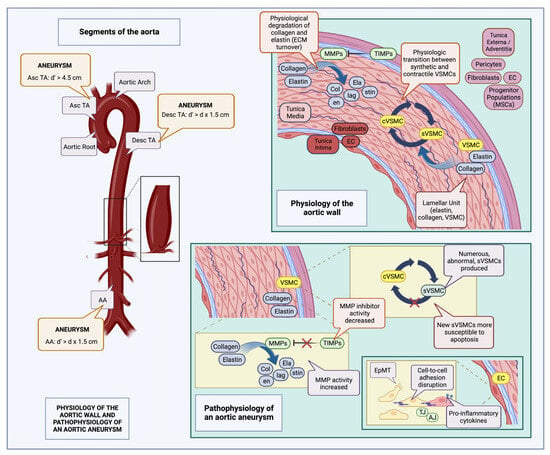
Figure 1. Physiology of the aortic wall and pathophysiology of an aortic aneurysm (created with BioRender.com): The aorta is composed of three layers, the tunica intima containing ECs along with fibroblasts in the subendothelial layer, the tunica media, composed of elastin interlaced with collagen, and VSMCs (lamellar units), and the tunica externa (adventitia), composed of pericytes, fibroblasts, ECs, and various progenitor cell populations, such as MSCs. Normally, there is a constant turnover of collagen and elastin fibers in the tunica media, carried out by MMPs and inhibited by TIMPs; there is also an equilibrium between synthetic (sVSMC) and contractile VSMC (cVSMC) populations [8,44,45]. Aneurysm definitions may vary according to location; thus, for the Asc TA, it is defined as a diameter (d’) exceeding 4.5 cm, while for the Desc TA, AA, as well as many visceral vessels, it is defined as an increase in diameter greater than the product of the initial vessel diameter multiplied by 1.5 (d’ > d x1.5) [8]. During aneurysm development, many of these normal physiological processes are disrupted. Abnormal sVSMCs, more susceptible to apoptosis, accumulate [36], there is increased MMP activity along with associated decreased TIMP activity, leading to augmented fragmentation of collagen and elastin fibers. In addition, EC intercellular adhesion is interrupted, due to AJ and TJ derangements, as well as switching of the EC phenotype, from an epithelial to a mesenchymal type; pro-inflammatory cytokines are also expressed on the EC surface, facilitating leukocyte entry into the aortic wall [41,46,47]. MSC: mesenchymal stem cells; TIMP: tissue inhibitors of metalloproteinase; MMP: matrix metalloproteinase; EC: endothelial cell; VSMC: vascular smooth muscle cell; TJ: tight junctions; AJ: adherens junctions; EpMT: epithelial-to-mesenchymal transition; Asc TA: ascending thoracic aorta; Desc TA: descending thoracic aorta; AA: abdominal aorta; d: initial aortic diameter; and d’: aneurysmal aortic diameter.
5. Current Treatment Strategies
5.1. Conservative Management
Aneurysms may be addressed conservatively with medical therapy and serial observation/follow-up, or they may be treated surgically. Conservative treatment generally aims to reduce growth rates, aneurysm-related mortality risk, and cardiovascular events, as well as ensuring a favorable post-operative outcome when used in conjunction with operative treatment [8,55]. Certain lifestyle modifications such as tobacco cessation and mild physical exercise may also have a positive effect on patients’ overall health [56,57,58]. On the other hand, while there is a scarcity of data regarding physical activity and dietary alterations, both are routinely recommended [55]. It is also worth noting here, the paradox associated with diabetes mellitus (DM) having a protective effect on aortic wall homeostasis; this might be due to modulation of factors associated with inflammation during states of hyperglycemia, though the exact pathophysiologic mechanisms have not been yet fully explored [59].
In order to alleviate the inflammation and associated shear stress on the aortic wall, blood pressure and cardiac contractility should be regulated [8,55]. Since uncontrolled hypertension poses a silent threat, which could lead to aortic dissection or even rupture, a blood pressure target of 130/80 mmHg is highly endorsed, though occasionally, a lower cut-off (<120 mmHg) is set if tolerated [60]. Finally, to further diminish the rate of potential cardiovascular adverse events and remodeling, as well as hinder aneurysm growth, statin uptake is also suggested [61].
Over time, with progressive dilation of the aneurysmal sac, an intraluminal thrombus may form [55]. The presence, and most importantly, the size of the thrombus, have both been implicated in further aneurysm progression and rupture; as such, initiation of an antithrombotic-antiplatelet regimen has been proposed [62,63], recommended for aortic aneurysms and also applicable to VAAs, such as renal artery aneurysms [63,64].
5.2. Criteria and Forms of Surgical Intervention
Repair in TAAs is usually indicated when the diameter reaches a size equal to or greater than 5.5 cm. However, in patients with genetic disease or other concomitant risk factors, intervention may be warranted at lower thresholds, for example, at 5 cm [8]. For AAAs on the other hand, repair is generally decided at a threshold equal to or greater than 5.5 cm for men and 5.0 cm for women [8].
Indications for surgical intervention in TAAA are not as clear cut as for AAAs, mostly due to a lack of appropriate, high-level evidence. In general, the presence or risk of rupture and acute dissection, the size of the aneurysm (diameter ≥ 6.0 cm, or ≥5.5 cm if carried out at specialized, multidisciplinary aortic centers), rapid growth > 1 cm, a penetrating atherosclerotic ulcer [8], and symptoms such as aortic valve insufficiency (when the ascending aorta is affected) are the main indications [9,65].
With regard to VAA treatment, for some, surgical repair is warranted once a certain diametric threshold is overcome if no other symptoms are present (for example, ≥3 cm for splenic artery aneurysms and ≥2 cm for celiac and jejunoileal artery aneurysms). For other VAA types, including pseudoaneurysms of any kind, as well as superior mesenteric (SMA) and colic artery aneurysms, surgical repair is usually indicated once diagnosis is confirmed [64].
Though open repair has been the most commonly utilized method for aortic and visceral aneurysm repair, it does not come without complications. Whereas massive hemorrhage, cardiac arrest, and multisystem organ failure might lead to death in extreme scenarios, there can also be debilitating complications that increase post-operative patient morbidity, such as paraplegia due to spinal cord ischemia and renal failure [66]. The advent of new techniques utilized in open repair however have considerably improved survival and reduced some of the associated post-operative complications [67,68]. An article published in 2022 elucidated the instances where open or endovascular repair might be favored, respectively. According to this, low-risk patients with unfavorable anatomy, chronic dissection, genetic disease, and appropriate cardiopulmonary reserve may be treated with open surgical repair. High-risk patients on the other hand, or patients with favorable aortic anatomy, may be appropriately tackled with endovascular methods [69,70].
Total endovascular repair (TEVAR) is an endovascular option for surgical treatment in both aortic aneurysms and VAAs [7,64]; endovascular devices used for this purpose may be multibranched, fenestrated, or both. The latter technique, termed FB-EVAR, i.e., fenestrated branched endovascular repair [71], when employed in the repair of aortic aneurysms, comprises a safe alternative method [72]. Though endovascular repair, compared to conventional open surgery, does exhibit better results in terms of morbidity and mortality, its potential complications warrant diligent attention and may demand reoperation. Apart from the possibility of endoleak, the stent itself may migrate, collapse, or become infected (in select cases of graft infection, mortality rates reach 50%), and its limbs may kink or occlude completely. There may also be systemic complications, including limb, renal, or other visceral organ ischemia as well as pelvic ischemia [73].
This entry is adapted from the peer-reviewed paper 10.3390/jcm12185878
This entry is offline, you can click here to edit this entry!
