You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Drought stress imposes substantial constraints on the growth and production of wheat (Triticum aestivum L.), a globally important cereal crop essential for food security. Plants have evolved various defense mechanisms to withstand environmental variations, such as stress escape, stress avoidance, and stress tolerance.
- drought stress
- wheat genotypes
- drought-resistant cultivars
1. Introduction
Plants have evolved various defense mechanisms to withstand environmental variations, such as stress escape, stress avoidance, and stress tolerance (Figure 1). These mechanisms can operate independently of adverse conditions or be specifically adapted to a particular stressor and facilitate a heritable plastic response [1]. By leveraging the stress-adaptive strategies, plants can better cope with extreme weather and improve their chances of survival and successful reproduction.
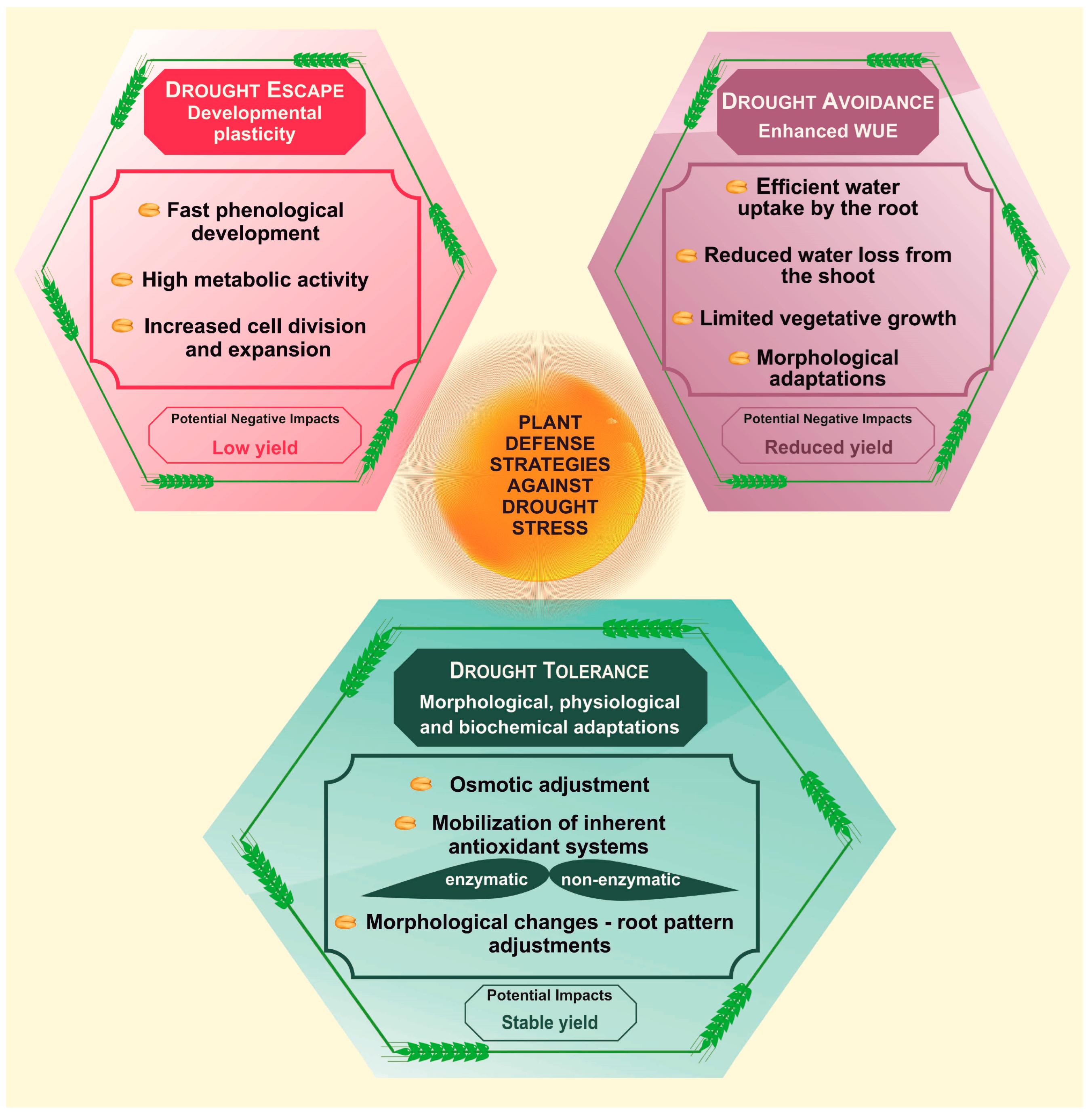
Figure 1. Plant defense mechanisms against drought stress. Abbreviations: WUE, water use efficiency.
2. Drought Escape Strategy
Drought escape is a survival mechanism that allows plants to successfully undergo their entire life cycle before experiencing drought-induced stress, entering a state of dormancy during periods of favorable weather conditions [2]. Drought-escaping plants typically do not undergo special morphological, physiological, or biochemical modifications. Instead, developmental plasticity is the key mechanism that enables these plants to escape from drought. Plant plasticity is associated with varying the duration of the transition from vegetative to reproductive stages, and fast phenological development, including early flowering and early maturation [3].
To achieve rapid plant development necessary for drought escape, several variables come into play, such as decreased stomatal conductance, low transpiration, and high photosynthetic carbon gain [4]. These factors promote metabolic activity, and lead to increased cell division and expansion and the formation of new seeds before the drought-induced end of the plant life cycle [5]. Drought escape strategy is widely employed by natural plants [6] and also by crops like wheat [2], rice [7], and Brassica rapa [4]. This strategy can be highly effective in coping with short or recurrent drought periods, but it may not be as efficient under prolonged water shortages [2]. This is because the strategy relies on plants completing their life cycle before the onset of drought stress, which can be challenging during prolonged drought periods.
Early flowering is a critical aspect of drought escape as it shortens the vegetative development phase, but it can potentially decrease crop yield. Therefore, the timing of the crop life cycle, primarily determined by the flowering time, is essential for yield production in water-limited environments. Under normal conditions, a longer crop cycle generally promotes productivity by allowing extended photosynthetic seasons and greater opportunities to harness solar energy [8]. Severe terminal drought can reduce yields due to depleted soil water before the end of the crop cycle [9]. For instance, wheat genotypes selected in Mediterranean environments, where terminal drought stress frequently occurs, show substantially lower production due to the higher risk of water stress during the reproductive or grain filling stages [10].
3. Drought Avoidance Strategy
Drought avoidance, also known as the ‘succulent strategy’, is a plant adaptation that enhances WUE in dry environments. This strategy can be achieved through two main mechanisms: efficient water uptake by the plant root system, and reduced water loss from the shoot parts [3]. Values of WUE may vary depending on plant species and the duration and severity of drought. A higher WUE is generally linked to increased yields under water shortage [11]. A drought avoidance strategy is typically connected with limited vegetative growth and involves specific plant adaptations, such as maintaining low metabolic rates, small or closed stomata to reduce water loss, decreased transpiration and photosynthesis rates, and morphological adjustments, like leaf curling and increased wax deposition on the leaf surface [12].
Plants that use a drought avoidance strategy can be broadly classified into two categories: water savers and water spenders [13]. Water savers limit water loss from the plant canopy by developing thicker cuticles, closing stomata, decreasing transpiration area and radiation absorption, and conserving water in specialized tissues for later use during grain filling and yield formation. In contrast, water spenders achieve a high tissue water status by maintaining water uptake through increased rooting and enhanced hydraulic conductance [14][15].
The drought avoidance strategy is evident not only in natural herbaceous populations [6] but also in cultivated crops [16][17]. Certain wheat cultivars possess specific attributes, such as thicker cuticles, reduced stomatal density, and deep root systems that allow them to minimize water loss through transpiration and survive in drought conditions [18][19]. Nevertheless, the effectiveness of this strategy depends on the interplay between plant adaptations, soil moisture conditions, and environmental variables. Although drought avoidance could be effective in conserving water, it is essential to bear in mind that it may potentially lead to limited vegetative growth, which could, in turn, reduce crop productivity.
4. Drought Tolerance Strategy
Drought tolerance is a complex phenomenon that enables plants to survive drought exposure by employing various physiological, biochemical, or morphological adaptations, which prevent or mitigate the harmful effects of dehydration [20]. This defense strategy involves drought-induced responses, encompassing physiological mechanisms that plants employ to contend with water scarcity across multiple tiers, spanning from the molecular to the whole-plant level. These mechanisms include the intricate regulation of gene expression, dynamic protein turnover, DNA or protein repair, the maintenance of tissue turgor through osmotic adjustment, alterations in cell wall extensibility, the reinforcement of protoplasmic drought resistance, the preservation of redox homeostasis, and the maintenance of tissue turgor. The latter involves osmotic adjustment, which entails the accumulation of soluble molecules to maintain cell turgor pressure [9][21]. These soluble compounds, commonly referred to as osmoprotectants, comprise substances like proline, polyamines, ammonium compounds, soluble sugars and various ions, all of which work to mitigate cell dehydration [22]. The accumulation of osmoprotectants results in a decrease in the inner osmotic potential of the cells, thereby enhancing water retention and maintaining homeostasis. These events ensure the proper functioning of plants under drought stress [23].
Water scarcity induces oxidative stress in plants due to the excessive generation of reactive oxygen species (ROS), which are byproducts resulting from the aerobic metabolism of plants. ROS can often trigger irreversible DNA damage and cell death, but they can also function as signaling molecules that regulate normal plant growth and stress responses [24][25]. To cope with challenging conditions, plants have developed mechanisms for drought tolerance, primarily relying on the mobilization of their endogenous antioxidant systems, which can alleviate the damaging effects [26]. These defense systems include enzymatic components, such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX) and glutathione reductase (GR), as well as nonenzymatic antioxidants like flavonoids, ascorbic acid, α-tocopherol, reduced glutathione and β-carotene [27]. Among enzymatic components, SOD efficiently converts ⋅OH into H2O2, and subsequently, the produced H2O2 is transformed into water and dioxygen by POX and CAT [28]. Non-enzymatic systems primarily rely on low-molecular-weight antioxidants such as glutathione, ascorbic acid and flavonoids, which eliminate hydroxyl radicals and singlet oxygen [29]. When cellular ROS levels exceed the capacity of these scavenging systems, cells enter an oxidative state, leading to oxidative modifications and potential cell damage, even culminating in cell death. Low ROS levels may act as second messengers in processes like stem cell maintenance, cell division, differentiation, organogenesis and responses to environmental factors [24][25]. Modulation of the antioxidant defense systems represents a major strategy for enhancing drought tolerance. Plant species resistant to dehydration generally contain higher concentrations of osmoprotectants and possess a more robust antioxidant system [26][30].
Drought-tolerant wheat cultivars have the capacity to sustain productivity even in water-limited conditions by employing various mechanisms, such as increased root growth, osmotic adjustment, and improved WUE to achieve drought tolerance [9][31][32][33][34][35], ensuring stable crop yield despite water scarcity. Selecting drought-tolerant wheat genotypes is not only economically viable but also a sustainable approach to supporting wheat production in drought-affected regions [34]. To facilitate the selection of such genotypes, it is essential to identify relevant plant features that confer advantages under drought stress. Screening for such traits, particularly during the early stages of wheat development, can provide valuable hints for the subsequent growth stages linked to plant productivity. Hence, wheat plants can employ a combination of drought escape, drought avoidance, and drought tolerance strategies. The choice of the most suitable strategy relies on the specific genetic makeup of the wheat cultivar and the prevailing environmental conditions.
In addition, the categorization of plants into drought-escape or drought-avoidance types is not entirely precise in practice because they occur at different times. Drought escape happens prior to a drought event, while drought avoidance occurs during or after a drought period. Researchers and breeders should actively pursue the development of wheat cultivars with a combination of strategies to increase their resilience to drought stress in changing environments.
This entry is adapted from the peer-reviewed paper 10.3390/agriculture13091823
References
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 2021, 11, 463.
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; De Groot, S.; Soole, K.; Langridge, P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950.
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, F1000 Faculty Rev-1554.
- Franks, S.J. Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytol. 2011, 190, 249–257.
- Sherrard, M.E.; Maherali, H. The adaptive significance of drought escape in Avena barbata, an annual grass. Evolution 2006, 60, 2478–2489.
- Kooyers, N.J. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci. 2015, 234, 155–162.
- Du, H.; Huang, F.; Wu, N.; Li, X.; Hu, H.; Xiong, L. Integrative regulation of drought escape through ABA dependent and independent pathways in rice. Mol. Plant. 2018, 11, 584–597.
- Dohleman, F.G.; Long, S.P. More productive than maize in the midwest: How does miscanthus do it? Plant Physiol. 2009, 150, 2104–2115.
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Annu. Rev. Plant Biol. 2018, 69, 733–759.
- Álvaro, F.; Isidro, J.; Villegas, D.; García del Moral, L.F.; Royo, C. Breeding effects on grain filling, biomass partitioning, and remobilization in Mediterranean durum wheat. Agron. J. 2008, 100, 361–370.
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103.
- Kim, K.S.; Park, S.H.; Jenks, M.A. Changes in leaf cuticular waxes of sesame (Sesamum indicum L.) plants exposed to water deficit. J. Plant Physiol. 2007, 164, 1134–1143.
- Delfin, E.F.; Drobnitch, S.T.; Comas, L.H. Plant strategies for maximizing growth during water stress and subsequent recovery in Solanum melongena L. (eggplant). PLoS ONE 2021, 16, e0256342.
- Mori, M.; Inagaki, M.N.; Inoue, T.; Nachit, M.M. Association of root water-uptake ability with drought adaptation in wheat. Cereal Res. Commun. 2011, 39, 551–559.
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384.
- Wang, J.Y.; Turner, N.C.; Liu, Y.X.; Kadambot, H.M.S.; Xiong, Y.C. Effects of drought stress on morphological, physiological and biochemical characteristics of wheat species differing in ploidy level. Funct. Plant Biol. 2016, 44, 219–234.
- Li, P.; Ma, B.; Palta, J.A.; Ding, T.; Cheng, Z.; Lv, G.; Xiong, Y. Wheat breeding highlights drought tolerance while ignores the advantages of drought avoidance: A meta-analysis. Eur. J. Agron. 2021, 122, 126196.
- Loss, S.P.; Siddique, K.H.M. Morphological and physiological traits associated with wheat yield increases in Mediterranean environments. Adv. Agron. 1994, 52, 229–276.
- Kulkarni, M.; Soolanayakanahally, R.; Ogawa, S.; Uga, Y.; Selvaraj, M.G.; Kagale, S. Drought response in wheat: Key genes and regulatory mechanisms controlling root system architecture and transpiration efficiency. Front. Chem. 2017, 5, 106.
- Puijalon, S.; Bouma, T.J.; Douady, C.J.; van Groenendael, J.; Anten, N.P.; Martel, E.; Bornette, G. Plant resistance to mechanical stress: Evidence of an avoidance-tolerance trade-off. New Phytol. 2011, 191, 1141–1149.
- Morgan, J.M. Osmoregulation as a selection criterion for drought tolerance in wheat. Aust. J. Agric. Res. 1983, 34, 607–614.
- Izanloo, A.; Condon, A.G.; Langridge, P.; Tester, M.; Schnurbusch, T. Different mechanisms of adaptation to cyclic water stress in two South Australian bread wheat cultivars. J. Exp. Bot. 2008, 59, 3327–3346.
- Farooq, M.; Hussain, M.; Siddique, K.H.M. Drought stress in wheat during flowering and grain-filling periods. CRC Crit. Rev. Plant Sci. 2014, 33, 331–349.
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800.
- Sharma, P.; Sharma, P.; Arora, P.; Verma, V.; Khanna, K.; Saini, P.; Bhardwaj, R. Role and regulation of ROS and antioxidants as signaling molecules in response to abiotic stresses. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 141–156.
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94.
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969.
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19.
- Todorova, D.; Sergiev, I.; Katerova, Z.; Shopova, E.; Dimitrova, L.; Brankova, L. Assessment of the biochemical responses of wheat seedlings to soil drought after application of selective herbicide. Plants 2021, 10, 733.
- Velinov, V.; Vaseva, I.; Zehirov, G.; Zhiponova, M.; Georgieva, M.; Vangheluwe, N.; Beeckman, T.; Vassileva, V. Overexpression of the NMig1 gene encoding a NudC domain protein enhances root growth and abiotic stress tolerance in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 815.
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168.
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10.
- Bengough, A.G.; McKenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 2011, 62, 59–68.
- Blum, A.; Jordan, W.R. Breeding crop varieties for stress environments. Crit. Rev. Plant Sci. 1985, 2, 199–238.
- Vassileva, V.; Signarbieux, C.; Anders, I.; Feller, U. Genotypic variation in drought stress response and subsequent recovery of wheat (Triticum aestivum L.). J. Plant Res. 2011, 124, 147–154.
This entry is offline, you can click here to edit this entry!
