Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Porous structure is an important three-dimensional morphological feature of the peripheral nerve guidance conduit (NGC), which permits the infiltration of cells, nutrients, and molecular signals and the discharge of metabolic waste. Porous structures with precisely customized pore sizes, porosities, and connectivities are being used to construct fully permeable, semi-permeable, and asymmetric peripheral NGCs for the replacement of traditional nerve autografts in the treatment of long-segment peripheral nerve injury.
- porous structure
- peripheral nerve guidance conduit
- peripheral nerve regeneration
1. Introduction
The application of tissue-engineered conduits in peripheral nerve regeneration has been extensively studied and successfully implemented in clinical practice [1]. Although the peripheral nervous system (PNS) possesses inherent regenerative capabilities, simple transverse or small-scale injuries can be effectively treated using epineural and/or fascicular sutures [2]. However, larger gaps in nerve segments (>3 mm) present a challenge, as axonal reconnection cannot occur. Autologous nerve grafting (typically from the sural nerve of the patient) has become the “gold standard” for treating long-gap nerve defects. Autologous cells provide a natural nerve structure that serves as a template for regeneration. Additionally, extracellular matrix proteins (ECMs), Schwann cells, and growth factors create an ideal microenvironment for peripheral nerve regeneration [3]. However, autologous grafts also have inherent disadvantages, including limited donors, loss of donor function, neuroma formation, nerve distortion or dislocation, and nerve diameter mismatch. Tissue reperfusion following autologous nerve transplantation induces apoptosis or necrosis. These limitations of autografts have prompted the development of alternative therapeutic options to improve patient outcomes [4].
Typically, NGCs are cylindrical tubular structures composed of degradable or nondegradable synthetic or natural materials that are implanted at the site of a nerve defect to facilitate specific regeneration of proximal axons towards their distal target organ [6]. Numerous artificial peripheral nerve repair grafts based on commercial products have been successfully implemented in clinical practice, including the Avance Nerve Graft (AxoGen, Inc., Alachua, FL, USA), NeuraWrapTM (Integra Life Sciences, Princeton, NI, USA), and NeuroFlex (Collagen Matrix, Princeton, NI, USA). With the development of neural tissue engineering and regenerative medicine, several ideal design requirements have emerged as guiding principles for continued nerve graft development [5]. NGCs in clinical application should have the following properties: (1) excellent bioactivity that can guide axons to grow from the proximal end into the distal stump, avoiding the formation of neuroma [7]; (2) mechanical performance to avoid wall breakage during suture and sufficient flexibility to avoid compression of neural tissue; (3) biocompatibility and nontoxicity to cells and tissue, with almost no immune response [8]; (4) a controllable degradation rate that matches the regeneration phase time of peripheral nerves, avoiding a second operation to remove undegraded implant materials and compression of the regenerated peripheral nerve by undegraded NGCs [9]; (5) a suitable porous structure that can limit the invasion of fibrous scarring into NGCs, hinder axon growth, and facilitate communication of biomolecule signals inside and outside the conduits and between cells and axons [10].
A porous structure of suitable pore size and connectivity was shown to be a determining factor for completion of the biological functions of NGCs, promoting the early adhesion, spreading, proliferation, and differentiation of Schwann cells to form cord-like structures (Bungner bands), promote vascularization, and reduce the formation of fibrous scars [11]. The concept of a nonpermeable silicone “nerve regeneration chamber” presented by Lundborg et al. [12] in the 1990s must be innovated, although its closed space can enrich bioactive factors (such as PDGF, FGF, and TGF-β secreted by Schwann cells or macrophages) and effectively prevent the invasion of fibrous scars. In vivo studies [13,14] have shown that the peripheral nerve repair effects of hollow impermeable conduits are outmatched by those of porous, permeable conduits. The 3D topology of the NGC directly affects the behavior of cells during nerve regeneration. The porosity and permeability of NGCs play important roles in the flow of oxygen, nutrients, and bioactive molecules between the internal and external environments [15]. Thus, porous structures with specific biological functions are critical for positive results in peripheral nerve repair. However, porous structures also have drawbacks; an excessively large pore size causes fibroblast deposition and hinders axon growth [2]. A lack of porosity affects the exchange efficiency of the internal and external walls of the conduits [11]. What type of porous structure should ideal NGCs have? What effect does the pore structure have on the physiological process of peripheral nerve regeneration? These questions do not seem to have clear answers from previous research. The transformation of peripheral nerve tissue repair materials in clinical applications can be promoted through a summary and analysis of the effects of porous structures on the regeneration and repair of NGCs.
As shown in Figure 1, this research reports the latest progress and applications of porous structures in peripheral-nerve-tissue-engineered conduits. The classification of NGCs according to pore structure characteristics and current methods of porosity measurement are summarized. The physical properties of the porous structure, including the biodegradability, mechanical performance, and permeability of NGCs, in addition to the biological behavior of cells related to peripheral nerve regeneration, are discussed.
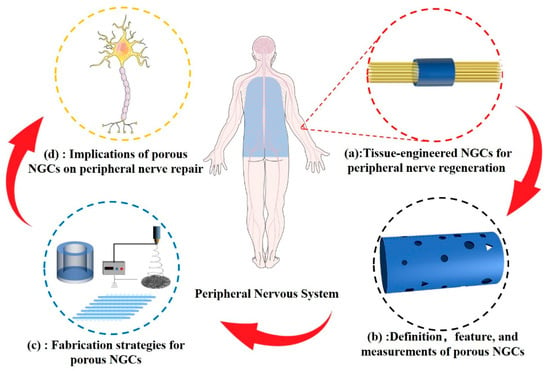
Figure 1. (a) Tissue-engineered NCGs represent a promising treatment strategy that can replace traditional autologous transplantation. (b) Definition, feature, and methods for measuring the porous structure of NGCs. (c) Strategies for porous NGC fabrication. (d) Comprehensive effects of porous NGCs on peripheral nerve regeneration.
2. Definition and Measurement of Pore Structure
The porous structure of an NGC refers to the three-dimensional morphology of closed or penetrating voids in biomaterials [16]. The pore structure is characterized by size, connectivity, uniformity, and three-dimensional morphology, which affect the biocompatibility, permeability, density, and mechanical performance of the NGC [11]. Generally, biomaterial pores can be categorized according to their size as macropores (100–500 μm), micropores (<100 μm), and nanopores (<1000 nm) [17]. The pore size of NGCs determines which molecules can be exchanged between the regenerated peripheral nerve and the surrounding microenvironment via the conduit walls. Depending on whether the cells can freely infiltrate the inside of the conduits, NGCs can be categorized as semi-permeable (<10 μm) or fully permeable (>50 μm) membranes [18]. Spherical, tubular, and irregularly shaped porous NGCs have been fabricated; however, with the development of new technologies, more complex structures with higher resolution will also be developed to meet the needs of peripheral nerve regeneration [19]. Pore morphology has attracted more and more attention in NGC design due to its significant influence on both the physiochemical and biological performances of the nerve graft. However, the pore morphology of porous conduits fabricated using traditional methods is usually random, and achieving special customization is difficult. Recently, with the development of lithography processes, electrospinning, and 3D printing technology, complex customized pore morphologies have been constructed. Chakkravarthy et al. [20] prepared a porous Ti-30Nb-2Zr biomimetic scaffold using 3D printing technology. In vitro cytological studies have shown that the pore structure can significantly promote cell adhesion, migration, and protein adsorption of the scaffold, possessing strong clinical application conversion ability. Based on these interconnections, pore structures can be divided into isolated and open structures. Almost all studies have considered open and penetrating pore structures; isolated and closed pore structures fail to achieve material exchange and reduce the mechanical performance of NGCs.
The pore size, porosity, permeability, and connectivity are important in characterizing the porous structure of NGCs, particularly in the development of innovative 3D topographies, as they play a decisive role in nerve regeneration. Conduits constructed from spun fibers are usually characterized by the fiber diameter and thickness. The morphology of porous NGCs can be observed through field emission scanning electron microscopy (SEM) and transmission electron microscopy (TEM); the pore size distribution can be statistically analyzed using image analysis software [16]. This method has been widely used to characterize porous biomaterials. Although it allows for intuitive observation of the 2D morphology of NGCs, it falls short of revealing the complete 3D morphology, and its objectivity is dependent on the observation site.
Porosity is defined as the void volume as a percentage of the total NGC volume [21]. The liquid displacement method in an ethanol medium has been widely used in recent studies. The main steps are described as follows [22]. The conduits are immersed in a known volume (V1) of anhydrous ethanol. Ethanol is forced into the pores of the conduit through a series of vacuum-release cycles, and the volume of the solution is recorded as V2. The conduits are removed, and the volume is recorded as V3. The NGC porosity is calculated using the following equation: porosity rate (%) = (V1 − V3)/(V2 − V3) × 100%. This method is simple and convenient, and it is not limited by the experimental equipment; however, the accuracy varies due to the volatility of ethanol, and closed pores cannot be measured. The gas adsorption method is an alternative for measuring the porosity and pore distribution of a conduit and is based on the adsorption of gas molecules on a solid surface [23]. The principle of this method is the exposure of a solid material to a gas environment at pressure, calculating the porosity and pore size distribution of the solid material by measuring the change in gas adsorption [24]. Both approaches have advantages and disadvantages. The liquid displacement method can measure larger pores but is insensitive to pores below the surface tension. The gas adsorption method is sensitive in measuring micropores but ignores the number of macropores and macroscale pores.
Permeability is primarily determined by the size and connectivity of the pore structure. NGCs with larger pores and high connectivity allow molecules and cells to in-fold and out-fold the tube walls more freely [11]. Waste products, such as cellular phospholipid debris due to Wallerian degeneration after peripheral nerve injury, should be released from conduits. Macrophages, vascular endothelial cells, fibroblasts, and Schwann cells gather at the site of peripheral nerve injury and participate in the regeneration of peripheral nerves by releasing bioactive signals via autocrine or paracrine pathways. Thus, the efficiency and mode of substance exchange inside and outside NGCs are important standards for evaluating the conduit repair function. Solutes with different molecular weights, including glucose (Mw 180 Da), lysozyme (Mw 14,600 Da), and BSA (Mw 62,000 Da), were used to simulate the osmotic flow dynamics of signal molecules with different molecular weights in the tube wall [25].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241814132
This entry is offline, you can click here to edit this entry!
