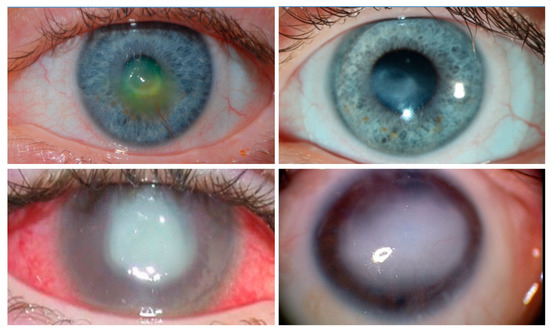
Corneal clarity is required for vision, and blindness occurs when the cornea becomes opaque. The cornea is covered by unique transparent epithelial cells that serve as an outermost cellular barrier bordering between the cornea and the external environment. Corneal sensory nerves protect the cornea from injury by triggering tearing and blink reflexes, and are also thought to regulate corneal epithelial renewal via unknown mechanism(s). When protective corneal sensory innervation is absent due to infection, trauma, intracranial tumors, surgery, or congenital causes, permanent blindness results from repetitive epithelial microtraumas and failure to heal. The condition is termed neurotrophic keratopathy (NK), with an incidence of 5:10,000 people worldwide.
- eyes
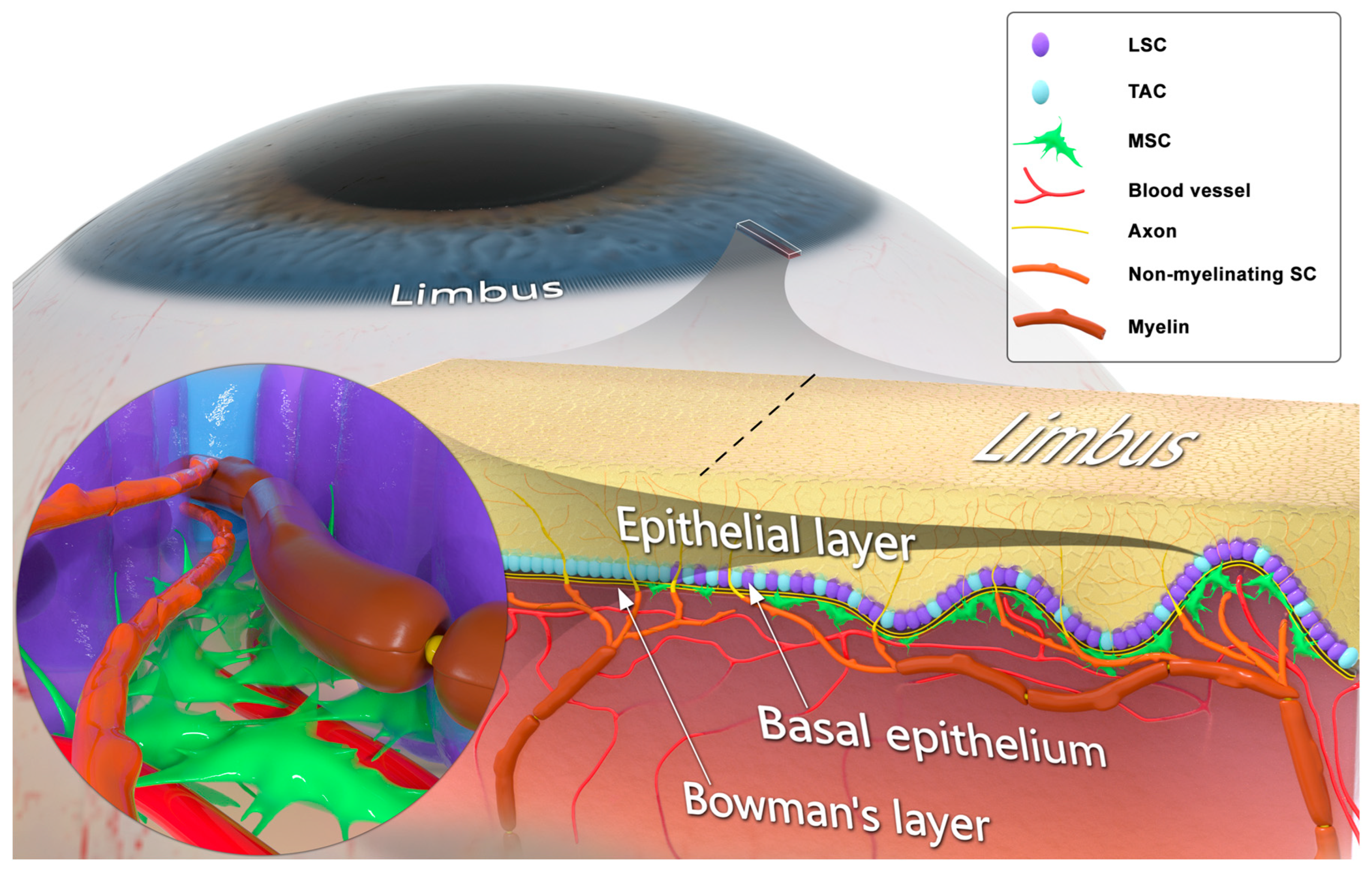
- limbus
- Schwann cells
- cornea
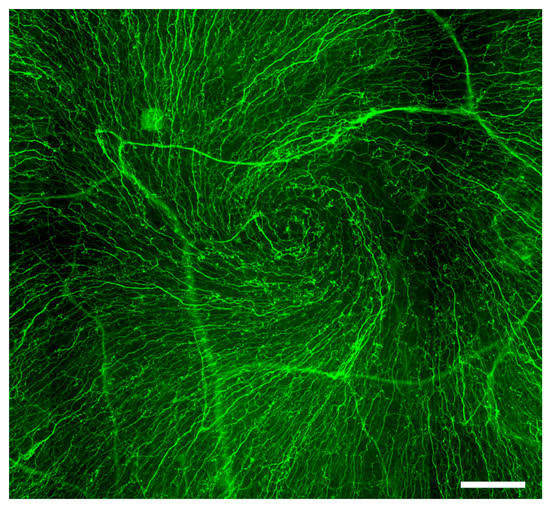
- corneal innervation
- neurotrophic keratopathy
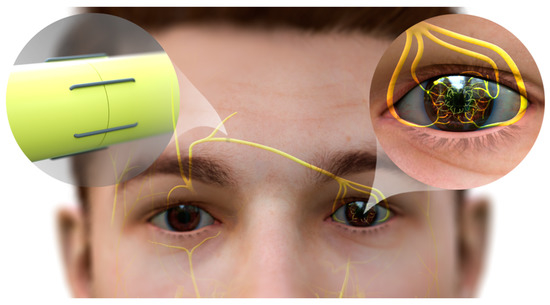
- corneal neurotization
- tacrolimus
- nerve growth factor
- cenegermin
1. Introduction
2. Corneal Sensory Innervation
3. Schwann Cells and Innervation-Dependent Corneal Epithelial Renewal
4. Trophic Regulation of Sensory Innervation-Mediated Corneal Epithelial Renewal
5. Currently Available Pharmacological Approaches to Treat NK
Nerve Growth Factor (NGF)
6. Strategies to Promote Corneal Reinnervation
6.1. Potential Treatment with Tacrolimus
6.2. Corneal Neurotization
This entry is adapted from the peer-reviewed paper 10.3390/ijms241612615
References
- Hertsenberg, A.J.; Funderburgh, J.L. Stem Cells in the Cornea. Prog. Mol. Biol. Transl. Sci. 2015, 134, 25–41.
- Hanna, C.; Bicknell, D.S.; O’Brien, J.E. Cell turnover in the adult human eye. Arch. Ophthalmol. 1961, 65, 695–698.
- Cenedella, R.J.; Fleschner, C.R. Kinetics of corneal epithelium turnover in vivo. Studies of lovastatin. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1957–1962.
- Bentley, A.J.; Nakamura, T.; Hammiche, A.; Pollock, H.M.; Martin, F.L.; Kinoshita, S.; Fullwood, N.J. Characterization of human corneal stem cells by synchrotron infrared micro-spectroscopy. Mol. Vis. 2007, 13, 237–242.
- Chen, Z.; de Paiva, C.S.; Luo, L.; Kretzer, F.L.; Pflugfelder, S.C.; Li, D.Q. Characterization of putative stem cell phenotype in human limbal epithelia. Stem. Cells 2004, 22, 355–366.
- Schlotzer-Schrehardt, U.; Kruse, F.E. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005, 81, 247–264.
- Romano, A.C.; Espana, E.M.; Yoo, S.H.; Budak, M.T.; Wolosin, J.M.; Tseng, S.C. Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5125–5129.
- Pellegrini, G.; Golisano, O.; Paterna, P.; Lambiase, A.; Bonini, S.; Rama, P.; De Luca, M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J. Cell Biol. 1999, 145, 769–782.
- Amin, S.; Jalilian, E.; Katz, E.; Frank, C.; Yazdanpanah, G.; Guaiquil, V.H.; Rosenblatt, M.I.; Djalilian, A.R. The Limbal Niche and Regenerative Strategies. Vision 2021, 5, 43.
- Yazdanpanah, G.; Haq, Z.; Kang, K.; Jabbehdari, S.; Rosenblatt, M.L.; Djalilian, A.R. Strategies for reconstructing the limbal stem cell niche. Ocul. Surf. 2019, 17, 230–240.
- Altshuler, A.; Amitai-Lange, A.; Tarazi, N.; Dey, S.; Strinkovsky, L.; Hadad-Porat, S.; Bhattacharya, S.; Nasser, W.; Imeri, J.; Ben-David, G.; et al. Discrete limbal epithelial stem cell populations mediate corneal homeostasis and wound healing. Cell Stem Cell 2021, 28, 1248–1261.e8.
- Li, W.; Hayashida, Y.; Chen, Y.T.; Tseng, S.C. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007, 17, 26–36.
- Cotsarelis, G.; Cheng, S.Z.; Dong, G.; Sun, T.T.; Lavker, R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell 1989, 57, 201–209.
- Zhao, J.; Mo, V.; Nagasaki, T. Distribution of label-retaining cells in the limbal epithelium of a mouse eye. J. Histochem. Cytochem. 2009, 57, 177–185.
- Pajoohesh-Ganji, A.; Pal-Ghosh, S.; Simmens, S.J.; Stepp, M.A. Integrins in slow-cycling corneal epithelial cells at the limbus in the mouse. Stem Cells 2006, 24, 1075–1086.
- Figueira, E.C.; Di Girolamo, N.; Coroneo, M.T.; Wakefield, D. The phenotype of limbal epithelial stem cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 144–156.
- Raji, B.; Dansault, A.; Leemput, J.; de la Houssaye, G.; Vieira, V.; Kobetz, A.; Arbogast, L.; Masson, C.; Menasche, M.; Abitbol, M. The RNA-binding protein Musashi-1 is produced in the developing and adult mouse eye. Mol. Vis. 2007, 13, 1412–1427.
- Thomas, P.B.; Liu, Y.H.; Zhuang, F.F.; Selvam, S.; Song, S.W.; Smith, R.E.; Trousdale, M.D.; Yiu, S.C. Identification of Notch-1 expression in the limbal basal epithelium. Mol. Vis. 2007, 13, 337–344.
- Di Girolamo, N.; Sarris, M.; Chui, J.; Cheema, H.; Coroneo, M.T.; Wakefield, D. Localization of the low-affinity nerve growth factor receptor p75 in human limbal epithelial cells. J. Cell Mol. Med. 2008, 12, 2799–2811.
- Barbaro, V.; Testa, A.; Di Iorio, E.; Mavilio, F.; Pellegrini, G.; De Luca, M. C/EBPdelta regulates cell cycle and self-renewal of human limbal stem cells. J. Cell Biol. 2007, 177, 1037–1049.
- Di Iorio, E.; Barbaro, V.; Ruzza, A.; Ponzin, D.; Pellegrini, G.; De Luca, M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 9523–9528.
- Amitai-Lange, A.; Altshuler, A.; Bubley, J.; Dbayat, N.; Tiosano, B.; Shalom-Feuerstein, R. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells 2015, 33, 230–239.
- Nasser, W.; Amitai-Lange, A.; Soteriou, D.; Hanna, R.; Tiosano, B.; Fuchs, Y.; Shalom-Feuerstein, R. Corneal-Committed Cells Restore the Stem Cell Pool and Tissue Boundary following Injury. Cell Rep. 2018, 22, 323–331.
- Farrelly, O.; Suzuki-Horiuchi, Y.; Brewster, M.; Kuri, P.; Huang, S.; Rice, G.; Bae, H.; Xu, J.; Dentchev, T.; Lee, V.; et al. Two-photon live imaging of single corneal stem cells reveals compartmentalized organization of the limbal niche. Cell Stem Cell 2021, 28, 1233–1247.e4.
- Mann, I. A Study of Epithelial Regeneration in the Living Eye. Br. J. Ophthalmol. 1944, 28, 26–40.
- Davanger, M.; Evensen, A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 1971, 229, 560–561.
- Pfister, R.R.; Burstein, N. The alkali burned cornea I. Epithelial and stromal repair. Exp. Eye. Res. 1976, 23, 519–535.
- Buck, R.C. Cell migration in repair of mouse corneal epithelium. Investig. Ophthalmol. Vis. Sci. 1979, 18, 767–784.
- Buck, R.C. Measurement of centripetal migration of normal corneal epithelial cells in the mouse. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1296–1299.
- Collinson, J.M.; Chanas, S.A.; Hill, R.E.; West, J.D. Corneal development, limbal stem cell function, and corneal epithelial cell migration in the Pax6+/− mouse. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1101–1108.
- Nagasaki, T.; Zhao, J. Centripetal movement of corneal epithelial cells in the normal adult mouse. Investig. Ophthalmol. Vis. Sci. 2003, 44, 558–566.
- Di Girolamo, N.; Bobba, S.; Raviraj, V.; Delic, N.C.; Slapetova, I.; Nicovich, P.R.; Halliday, G.M.; Wakefield, D.; Whan, R.; Lyons, J.G. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells 2015, 33, 157–169.
- Peterson, D.C.; Hamel, R.N. Corneal Reflex. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Muller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542.
- Ueno, H.; Ferrari, G.; Hattori, T.; Saban, D.R.; Katikireddy, K.R.; Chauhan, S.K.; Dana, R. Dependence of corneal stem/progenitor cells on ocular surface innervation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 867–872.
- Cavanagh, H.D.; Colley, A.M. The molecular basis of neurotrophic keratitis. Acta Ophthalmol. Suppl. 1989, 192, 115–134.
- Bonini, S.; Rama, P.; Olzi, D.; Lambiase, A. Neurotrophic keratitis. Eye 2003, 17, 989–995.
- Sigelman, S.; Friedenwald, J.S. Mitotic and wound-healing activities of the corneal epithelium; effect of sensory denervation. AMA Arch. Ophthalmol. 1954, 52, 46–57.
- Nakamura, M.; Nishida, T.; Ofuji, K.; Reid, T.W.; Mannis, M.J.; Murphy, C.J. Synergistic effect of substance P with epidermal growth factor on epithelial migration in rabbit cornea. Exp. Eye Res. 1997, 65, 321–329.
- Yamada, N.; Yanai, R.; Inui, M.; Nishida, T. Sensitizing effect of substance P on corneal epithelial migration induced by IGF-1, fibronectin, or interleukin-6. Investig. Ophthalmol. Vis. Sci. 2005, 46, 833–839.
- Murphy, C.J.; Marfurt, C.F.; McDermott, A.; Bentley, E.; Abrams, G.A.; Reid, T.W.; Campbell, S. Spontaneous chronic corneal epithelial defects (SCCED) in dogs: Clinical features, innervation, and effect of topical SP, with or without IGF-1. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2252–2261.
- Marfurt, C.F.; Echtenkamp, S.F. The effect of diabetes on neuropeptide content in the rat cornea and iris. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1100–1106.
- Elbadri, A.A.; Shaw, C.; Johnston, C.F.; Archer, D.B.; Buchanan, K.D. The distribution of neuropeptides in the ocular tissues of several mammals: A comparative study. Comp. Biochem. Physiol. C 1991, 100, 625–627.
- Yang, L.; Di, G.; Qi, X.; Qu, M.; Wang, Y.; Duan, H.; Danielson, P.; Xie, L.; Zhou, Q. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes 2014, 63, 4262–4274.
- Marfurt, C.F.; Kingsley, R.E.; Echtenkamp, S.E. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Investig. Ophthalmol. Vis. Sci. 1989, 30, 461–472.
- Jones, M.A.; Marfurt, C.F. Calcitonin gene-related peptide and corneal innervation: A developmental study in the rat. J. Comp. Neurol. 1991, 313, 132–150.
- Garcia-Hirschfeld, J.; Lopez-Briones, L.G.; Belmonte, C. Neurotrophic influences on corneal epithelial cells. Exp. Eye Res. 1994, 59, 597–605.
- Lambiase, A.; Rama, P.; Bonini, S.; Caprioglio, G.; Aloe, L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N. Engl. J. Med. 1998, 338, 1174–1180.
- Lambiase, A.; Manni, L.; Bonini, S.; Rama, P.; Micera, A.; Aloe, L. Nerve growth factor promotes corneal healing: Structural, biochemical, and molecular analyses of rat and human corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1063–1069.
- Tan, M.H.; Bryars, J.; Moore, J. Use of nerve growth factor to treat congenital neurotrophic corneal ulceration. Cornea 2006, 25, 352–355.
- Ivanusic, J.J.; Wood, R.J.; Brock, J.A. Sensory and sympathetic innervation of the mouse and guinea pig corneal epithelium. J. Comp. Neurol. 2013, 521, 877–893.
- Alper, M.G. The anesthetic eye: An investigation of changes in the anterior ocular segment of the monkey caused by interrupting the trigeminal nerve at various levels along its course. Trans. Am. Ophthalmol. Soc. 1975, 73, 323–365.
- Beuerman, R.W.; Schimmelpfennig, B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp. Neurol. 1980, 69, 196–201.
- Sacchetti, M.; Lambiase, A. Diagnosis and management of neurotrophic keratitis. Clin. Ophthalmol. 2014, 8, 571–579.
- Dana, R.; Farid, M.; Gupta, P.K.; Hamrah, P.; Karpecki, P.; McCabe, C.M.; Nijm, L.; Pepose, J.S.; Pflugfelder, S.; Rapuano, C.J.; et al. Expert consensus on the identification, diagnosis, and treatment of neurotrophic keratopathy. BMC Ophthalmol. 2021, 21, 327.
- Schimmelpfennig, B.; Beuerman, R. A technique for controlled sensory denervation of the rabbit cornea. Graefes. Arch. Clin. Exp. Ophthalmol. 1982, 218, 287–293.
- Araki, K.; Ohashi, Y.; Kinoshita, S.; Hayashi, K.; Kuwayama, Y.; Tano, Y. Epithelial wound healing in the denervated cornea. Curr. Eye Res. 1994, 13, 203–211.
- Gallar, J.; Pozo, M.A.; Rebollo, I.; Belmonte, C. Effects of capsaicin on corneal wound healing. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1968–1974.
- Ferrari, G.; Chauhan, S.K.; Ueno, H.; Nallasamy, N.; Gandolfi, S.; Borges, L.; Dana, R. A novel mouse model for neurotrophic keratopathy: Trigeminal nerve stereotactic electrolysis through the brain. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2532–2539.
- Ramaesh, K.; Stokes, J.; Henry, E.; Dutton, G.N.; Dhillon, B. Congenital corneal anesthesia. Surv. Ophthalmol. 2007, 52, 50–60.
- Rosenberg, M.L. Congenital trigeminal anaesthesia. A review and classification. Brain 1984, 107 Pt 4, 1073–1082.
- Lambley, R.G.; Pereyra-Munoz, N.; Parulekar, M.; Mireskandari, K.; Ali, A. Structural and functional outcomes of anaesthetic cornea in children. Br. J. Ophthalmol. 2015, 99, 418–424.
- Agranat, J.S.; Kitos, N.R.; Jacobs, D.S. Prosthetic replacement of the ocular surface ecosystem: Impact at 5 years. Br. J. Ophthalmol. 2016, 100, 1171–1175.
- Rozsa, A.J.; Beuerman, R.W. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain 1982, 14, 105–120.
- Utsunomiya, T.; Nagaoka, T.; Hanada, K.; Omae, T.; Yokota, H.; Abiko, A.; Haneda, M.; Yoshida, A. Imaging of the Corneal Subbasal Whorl-like Nerve Plexus: More Accurate Depiction of the Extent of Corneal Nerve Damage in Patients With Diabetes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5417–5423.
- Adachi, W.; Ulanovsky, H.; Li, Y.; Norman, B.; Davis, J.; Piatigorsky, J. Serial analysis of gene expression (SAGE) in the rat limbal and central corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3801–3810.
- Mirmoeini, K.; Tajdaran, K.; Zhang, J.; Gordon, T.; Ali, A.; Kaplan, D.R.; Feinberg, K.; Borschel, G.H. Schwann Cells are Key Regulators of Corneal Epithelial Renewal. IOVS 2023, 64, 7.
- Johnston, A.P.; Yuzwa, S.A.; Carr, M.J.; Mahmud, N.; Storer, M.A.; Krause, M.P.; Jones, K.; Paul, S.; Kaplan, D.R.; Miller, F.D. Dedifferentiated Schwann Cell Precursors Secreting Paracrine Factors Are Required for Regeneration of the Mammalian Digit Tip. Cell Stem Cell 2016, 19, 433–448.
- Johnston, A.P.; Naska, S.; Jones, K.; Jinno, H.; Kaplan, D.R.; Miller, F.D. Sox2-mediated regulation of adult neural crest precursors and skin repair. Stem Cell Rep. 2013, 1, 38–45.
- Yamazaki, S.; Ema, H.; Karlsson, G.; Yamaguchi, T.; Miyoshi, H.; Shioda, S.; Taketo, M.M.; Karlsson, S.; Iwama, A.; Nakauchi, H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 2011, 147, 1146–1158.
- Semeraro, F.; Forbice, E.; Romano, V.; Angi, M.; Romano, M.R.; Filippelli, M.E.; Di Iorio, R.; Costagliola, C. Neurotrophic keratitis. Ophthalmologica 2014, 231, 191–197.
- Labetoulle, M.; Baudouin, C.; Calonge, M.; Merayo-Lloves, J.; Boboridis, K.G.; Akova, Y.A.; Aragona, P.; Geerling, G.; Messmer, E.M.; Benitez-Del-Castillo, J. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019, 97, 137–145.
- Shimizu, T.; Izumi, K.; Fujita, S.; Koja, T.; Sorimachi, M.; Ohba, N.; Fukuda, T. Capsaicin-induced corneal lesions in mice and the effects of chemical sympathectomy. J. Pharmacol. Exp. Ther. 1987, 243, 690–695.
- Yamada, M.; Ogata, M.; Kawai, M.; Mashima, Y. Decreased substance P concentrations in tears from patients with corneal hypesthesia. Am. J. Ophthalmol. 2000, 129, 671–672.
- Nishida, T.; Tanaka, T. Extracellular matrix and growth factors in corneal wound healing. Curr. Opin. Ophthalmol. 1996, 7, 2–11.
- Reid, T.W.; Murphy, C.J.; Iwahashi, C.K.; Foster, B.A.; Mannis, M.J. Stimulation of epithelial cell growth by the neuropeptide substance P. J. Cell Biochem. 1993, 52, 476–485.
- Kruse, F.E.; Tseng, S.C. Growth factors modulate clonal growth and differentiation of cultured rabbit limbal and corneal epithelium. Investig. Ophthalmol. Vis. Sci. 1993, 34, 1963–1976.
- Ghalibafan, S.; Osei, K.; Amescua, G.; Sabater, A. Efficacy of Plasma Rich in Growth Factors (PRGF) in Stage 1 Neurotrophic Keratitis. Res. Sq. 2023, 64, 5151.
- Castro Mora, M.P.; Palacio Varona, J.; Perez Riano, B.; Laverde Cubides, C.; Rey-Rodriguez, D.V. Effectiveness of topical insulin for the treatment of surface corneal pathologies. Arch. Soc. Esp. Oftalmol. (Engl. Ed.) 2023, 98, 220–232.
- Wang, A.L.; Weinlander, E.; Metcalf, B.M.; Barney, N.P.; Gamm, D.M.; Nehls, S.M.; Struck, M.C. Use of Topical Insulin to Treat Refractory Neurotrophic Corneal Ulcers. Cornea 2017, 36, 1426–1428.
- Pflugfelder, S.C.; Massaro-Giordano, M.; Perez, V.L.; Hamrah, P.; Deng, S.X.; Espandar, L.; Foster, C.S.; Affeldt, J.; Seedor, J.A.; Afshari, N.A.; et al. Topical Recombinant Human Nerve Growth Factor (Cenegermin) for Neurotrophic Keratopathy: A Multicenter Randomized Vehicle-Controlled Pivotal Trial. Ophthalmology 2020, 127, 14–26.
- Bonini, S.; Lambiase, A.; Rama, P.; Sinigaglia, F.; Allegretti, M.; Chao, W.; Mantelli, F.; Group, R.S. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology 2018, 125, 1332–1343.
- Kolli, S.; Bojic, S.; Ghareeb, A.E.; Kurzawa-Akanbi, M.; Figueiredo, F.C.; Lako, M. The Role of Nerve Growth Factor in Maintaining Proliferative Capacity, Colony-Forming Efficiency, and the Limbal Stem Cell Phenotype. Stem Cells 2019, 37, 139–149.
- Deeks, E.D.; Lamb, Y.N. Cenegermin: A Review in Neurotrophic Keratitis. Drugs 2020, 80, 489–494.
- Sheha, H.; Tighe, S.; Hashem, O.; Hayashida, Y. Update On Cenegermin Eye Drops In The Treatment Of Neurotrophic Keratitis. Clin. Ophthalmol. 2019, 13, 1973–1980.
- Blanco-Mezquita, T.; Martinez-Garcia, C.; Proenca, R.; Zieske, J.D.; Bonini, S.; Lambiase, A.; Merayo-Lloves, J. Nerve growth factor promotes corneal epithelial migration by enhancing expression of matrix metalloprotease-9. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3880–3890.
- Bonini, S.; Lambiase, A.; Rama, P.; Caprioglio, G.; Aloe, L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology 2000, 107, 1347–1351, Discussion 1351—1352.
- Donnerer, J.; Amann, R.; Schuligoi, R.; Skofitsch, G. Complete recovery by nerve growth factor of neuropeptide content and function in capsaicin-impaired sensory neurons. Brain Res. 1996, 741, 103–108.
- Shoughy, S.S. Topical tacrolimus in anterior segment inflammatory disorders. Eye Vis. 2017, 4, 7.
- Erdinest, N.; Ben-Eli, H.; Solomon, A. Topical tacrolimus for allergic eye diseases. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 535–543.
- Magalhaes, O.A.; Marinho, D.R.; Kwitko, S. Topical 0.03% tacrolimus preventing rejection in high-risk corneal transplantation: A cohort study. Br. J. Ophthalmol. 2013, 97, 1395–1398.
- Lyons, W.E.; George, E.B.; Dawson, T.M.; Steiner, J.P.; Snyder, S.H. Immunosuppressant FK506 promotes neurite outgrowth in cultures of PC12 cells and sensory ganglia. Proc. Natl. Acad. Sci. USA 1994, 91, 3191.
- Gold, B.G.; Densmore, V.; Shou, W.; Matzuk, M.M.; Gordon, H.S. Immunophilin FK506-binding protein 52 (not FK506-binding protein 12) mediates the neurotrophic action of FK506. J. Pharmacol. Exp. Ther. 1999, 289, 1202–1210.
- Gold, B.G.; Yew, J.Y.; Zeleny-Pooley, M. The immunosuppressant FK506 increases GAP-43 mRNA levels in axotomized sensory neurons. Neurosci. Lett. 1998, 241, 25–28.
- Steiner, J.P.; Connolly, M.A.; Valentine, H.L.; Hamilton, G.S.; Dawson, T.M.; Hester, L.; Snyder, S.H. Neurotrophic actions of nonimmunosuppressive analogues of immunosuppressive drugs FK506, rapamycin and cyclosporin A. Nat. Med. 1997, 3, 421–428.
- Yang, R.K.; Lowe, J.B., 3rd; Sobol, J.B.; Sen, S.K.; Hunter, D.A.; Mackinnon, S.E. Dose-dependent effects of FK506 on neuroregeneration in a rat model. Plast. Reconstr. Surg. 2003, 112, 1832–1840.
- Iwasaki, K.; Shiraga, T.; Matsuda, H.; Teramura, Y.; Kawamura, A.; Hata, T.; Ninomiya, S.; Esumi, Y. Absorption, Distribution, Metabolism and Excretion of Tacrolimus (FK506) in the Rat. Drug Metab. Pharmacokinet. 1998, 13, 259–265.
- Gold, B.G.; Storm-Dickerson, T.; Austin, D.R. The immunosuppressant FK506 increases functional recovery and nerve regeneration following peripheral nerve injury. Restor. Neurol. Neurosci. 1994, 6, 287–296.
- Sulaiman, O.A.; Voda, J.; Gold, B.G.; Gordon, T. FK506 increases peripheral nerve regeneration after chronic axotomy but not after chronic schwann cell denervation. Exp. Neurol. 2002, 175, 127–137.
- Jo, S.; Pan, D.; Halevi, A.E.; Roh, J.; Schellhardt, L.; Hunter Ra, D.A.; Snyder-Warwick, A.K.; Moore, A.M.; Mackinnon, S.E.; Wood, M.D. Comparing electrical stimulation and tacrolimus (FK506) to enhance treating nerve injuries. Muscle Nerve 2019, 60, 629–636.
- Daneri-Becerra, C.; Patiño-Gaillez, M.G.; Galigniana, M.D. Proof that the high molecular weight immunophilin FKBP52 mediates the in vivo neuroregenerative effect of the macrolide FK506. Biochem. Pharmacol. 2020, 182, 114204.
- Quintá, H.R.; Galigniana, M.D. The neuroregenerative mechanism mediated by the Hsp90-binding immunophilin FKBP52 resembles the early steps of neuronal differentiation. Br. J. Pharmacol. 2012, 166, 637–649.
- Shim, S.; Yuan, J.P.; Kim, J.Y.; Zeng, W.; Huang, G.; Milshteyn, A.; Kern, D.; Muallem, S.; Ming, G.-l.; Worley, P.F. Peptidyl-Prolyl Isomerase FKBP52 Controls Chemotropic Guidance of Neuronal Growth Cones via Regulation of TRPC1 Channel Opening. Neuron 2009, 64, 471–483.
- Udina, E.; Ceballos, D.; Verdú, E.; Gold, B.G.; Navarro, X. Bimodal dose-dependence of FK506 on the rate of axonal regeneration in mouse peripheral nerve. Muscle Nerve 2002, 26, 348–355.
- Gold, B.G.; Katoh, K.; Storm-Dickerson, T. The immunosuppressant FK506 increases the rate of axonal regeneration in rat sciatic nerve. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 7509–7516.
- Zuo, K.J.; Shafa, G.; Chan, K.; Zhang, J.; Hawkins, C.; Tajdaran, K.; Gordon, T.; Borschel, G.H. Local FK506 drug delivery enhances nerve regeneration through fresh, unprocessed peripheral nerve allografts. Exp. Neurol. 2021, 341, 113680.
- Tajdaran, K.; Shoichet, M.S.; Gordon, T.; Borschel, G.H. A novel polymeric drug delivery system for localized and sustained release of tacrolimus (FK506). Biotechnol. Bioeng. 2015, 112, 1948–1953.
- Tajdaran, K.; Chan, K.; Shoichet, M.S.; Gordon, T.; Borschel, G.H. Local delivery of FK506 to injured peripheral nerve enhances axon regeneration after surgical nerve repair in rats. Acta Biomater. 2019, 96, 211–221.
- Islam, F.; Westcott, M.; Rees, A.; Robson, A.G.; Kapoor, B.; Holder, G.; Pavesio, C. Safety profile and efficacy of tacrolimus in the treatment of birdshot retinochoroiditis: A retrospective case series review. Br. J. Ophthalmol. 2018, 102, 983–990.
- Ghaffari, R.; Ghassemi, H.; Zarei-Ghanavati, M.; Latifi, G.; Dehghani, S.; Haq, Z.; Djalilian, A.R. Tacrolimus Eye Drops as Adjunct Therapy in Severe Corneal Endothelial Rejection Refractory to Corticosteroids. Cornea 2017, 36, 1195–1199.
- Shoughy, S.S.; Jaroudi, M.O.; Tabbara, K.F. Efficacy and safety of low-dose topical tacrolimus in vernal keratoconjunctivitis. Clin. Ophthalmol. 2016, 10, 643–647.
- Paller, A.S.; Fölster-Holst, R.; Chen, S.C.; Diepgen, T.L.; Elmets, C.; Margolis, D.J.; Pollock, B.H. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J. Am. Acad. Dermatol. 2020, 83, 375–381.
- Bentata, Y. Tacrolimus: 20 years of use in adult kidney transplantation. What we should know about its nephrotoxicity. Artif. Organs 2020, 44, 140–152.
- Cury Martins, J.; Martins, C.; Aoki, V.; Gois, A.F.; Ishii, H.A.; da Silva, E.M. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst. Rev. 2015, 2015, Cd009864.
- Provenzani, A.; Santeusanio, A.; Mathis, E.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Provenzani, A.; Polidori, C.; Vizzini, G.; Polidori, P.; et al. Pharmacogenetic considerations for optimizing tacrolimus dosing in liver and kidney transplant patients. World J. Gastroenterol. 2013, 19, 9156–9173.
- Böttiger, Y.; Brattström, C.; Tydén, G.; Säwe, J.; Groth, C.G. Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. Br. J. Clin. Pharmacol. 1999, 48, 445–448.
- Luaces-Rodríguez, A.; Touriño-Peralba, R.; Alonso-Rodríguez, I.; García-Otero, X.; González-Barcia, M.; Rodríguez-Ares, M.T.; Martínez-Pérez, L.; Aguiar, P.; Gómez-Lado, N.; Silva-Rodríguez, J.; et al. Preclinical characterization and clinical evaluation of tacrolimus eye drops. Eur. J. Pharm. Sci. 2018, 120, 152–161.
- Catapano, J.; Fung, S.S.M.; Halliday, W.; Jobst, C.; Cheyne, D.; Ho, E.S.; Zuker, R.M.; Borschel, G.H.; Ali, A. Treatment of neurotrophic keratopathy with minimally invasive corneal neurotisation: Long-term clinical outcomes and evidence of corneal reinnervation. Br. J. Ophthalmol. 2019, 103, 1724–1731.
- Terzis, J.K.; Dryer, M.M.; Bodner, B.I. Corneal neurotization: A novel solution to neurotrophic keratopathy. Plast. Reconstr. Surg. 2009, 123, 112–120.
- Elbaz, U.; Bains, R.; Zuker, R.M.; Borschel, G.H.; Ali, A. Restoration of corneal sensation with regional nerve transfers and nerve grafts: A new approach to a difficult problem. JAMA Ophthalmol. 2014, 132, 1289–1295.
- Woo, J.H.; Daeschler, S.C.; Mireskandari, K.; Borschel, G.H.; Ali, A. Minimally Invasive Corneal Neurotization Provides Sensory Function, Protects Against Recurrent Ulceration, and Improves Visual Acuity. Am. J. Ophthalmol. 2022, 241, 179–189.
