Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Soft tissue regeneration holds significant promise for addressing various clinical challenges, ranging from craniofacial and oral tissue defects to blood vessels, muscle, and fibrous tissue regeneration. Mesenchymal stem cells (MSCs) have emerged as a promising tool in regenerative medicine due to their unique characteristics and potential to differentiate into multiple cell lineages.
- mesenchymal stem cells
- regenerative medicine
- tissue regeneration
- tissue engineering
1. Introduction
The repair and restoration of different tissues throughout the human body is a complex and dynamic process, crucial for maintaining the body’s overall integrity and function in response to various injuries and disease states [1][2]. Soft tissues, including skin, muscles, blood vessels, nerves, and fibrous tissues, play diverse roles and contribute to the body’s integrity and functionality [3]. These tissues are involved in essential functions such as temperature regulation, locomotion, oxygen and nutrient transport, sensory perception, and structural support.
Traditional therapeutic approaches for soft tissue injuries or degenerative disorders have relied on conservative therapies such as physical therapy, medication, and surgery. While these methods offer some benefits like comfort and tissue healing, they often have limitations [4]. Severe soft tissue injuries may surpass the body’s innate regenerative capacity, leading to inadequate repair. Scar tissue formation during healing can impede proper tissue functionality and slow recovery. Furthermore, conventional treatments may struggle to restore tissue structure and function, especially in complex soft tissue environments with diverse cell types and intricate tissue architectures [5].
In recent years, regenerative medicine has emerged as a promising field aiming to overcome the limitations of traditional treatments and provide innovative approaches for soft tissue repair and regeneration [5]. Regenerative medicine is a broad term that encompasses a range of strategies, such as tissue engineering, cellular therapy, gene therapy, and the use of biomaterials, to restore damaged or diseased tissues to their original form and function [6][7][8]. Out of cell-based therapies, mesenchymal stem cells (MSCs) have garnered significant attention due to their unique characteristics and regenerative potential. MSCs possess self-renewal and differentiation capabilities, including the ability of MSCs to differentiate into cell types specific to soft tissues. This ability makes MSCs a particularly promising avenue for soft tissue regeneration [9].
2. Mesenchymal Stem Cells
MSCs are a type of multipotent stem cell derived from various sources, such as bone marrow (BM-MSCs), adipose tissue (AD-MSCs), umbilical cord (UC-MSCs), and dental pulp (DP-MSCs) [10]. These cells have gained attention in regenerative medicine due to their ability to self-renew, modulate the immune response, and differentiate into multiple cell lineages. Their differentiation potential into fibroblasts, chondrocytes, osteoblasts, and adipocytes makes them particularly attractive for tissue repair and regeneration [11].
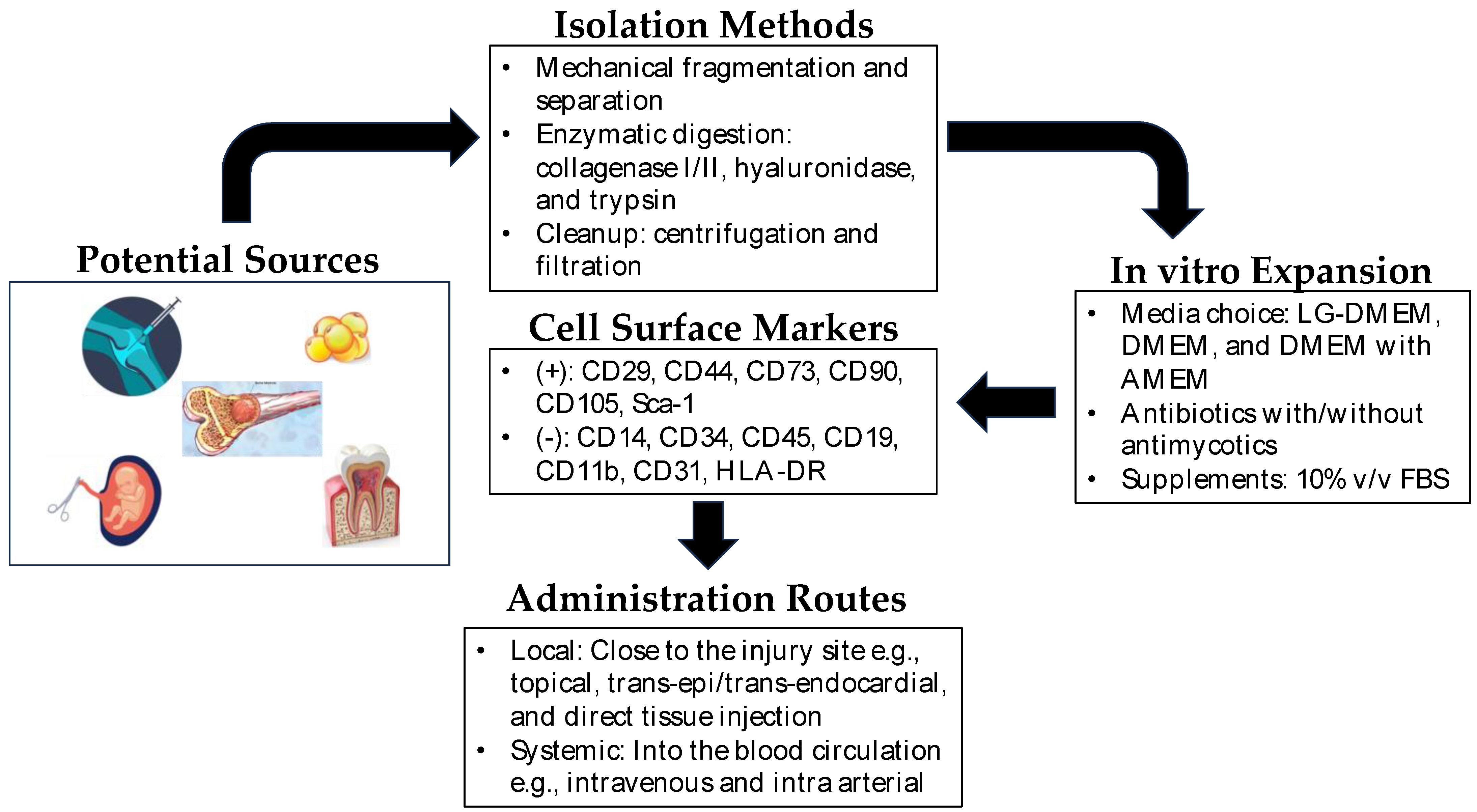
The extraction methods for MSCs vary depending on the tissue source. For example, BM-MSCs are typically harvested from bone marrow aspiration, followed by isolation and expansion in culture. AD-MSCs are obtained through liposuction or surgical excision of adipose tissue, which is then processed to extract the MSCs. UC-MSCs are derived from umbilical cord tissue or blood, while DP-MSCs are obtained from dental pulp extracted for therapeutic or orthodontic purposes [12]. Each source has its advantage and disadvantage as cell yield, proliferation capacity, and differentiation potential, which should be considered based on specific applications. Figure 1 shows the general scheme for the isolation and expansion of MSCs for clinical applications.

Figure 1. Mesenchymal stem cells in regenerative medicine. Depicted above is a brief overview of the isolation, expansion, and administration of mesenchymal stem cells in regenerative therapy. Potential sources of MSCs include synovium, bone marrow, dental pulp, amniotic fluid, umbilical cord, and adipose tissue. The isolation of MSCs from these sources is very straightforward, and MSCs are easily expandable in vitro. In clinical settings, they can be administered locally near the site of the injured tissue or directly into the blood (intravenous/intra-arterial) as systemic administration.
Characterizing MSCs is essential to confirm their identity and quality. The International Society for Cellular Therapy (ISCT) has established minimal criteria to define MSCs, including adherence to plastic, expression of specific surface markers (CD73, CD90, CD105), and absence of hematopoietic markers (CD45, CD34, CD14, or CD11b, CD79a, or CD19, HLA-DR) [13].
MSCs have already received approval from the U.S. Food and Drug Administration (FDA) for some clinical applications. For instance, BM-MSCs are used to treat complications arising from hematopoietic stem cell transplantation, such as graft-versus-host disease (GVHD) [14]. BM-MSCs have immunomodulatory properties, suppressing the immune response and promoting tissue repair. Promising results from clinical trials led to FDA approval of MSC-based therapies, and ongoing investigations explore their potential in cartilage and bone repair, wound healing, and autoimmune disorders [9].
2.1. MSCs in Craniofacial and Oral Soft Tissue Regeneration
Craniofacial and oral soft tissue regeneration aims to restore damaged or lost tissues in the head and face region resulting from trauma, congenital conditions, surgical interventions, or cancer resections [15]. This anatomically complex area includes craniofacial districts such as the maxillofacial region, oral cavity, mandibular area, and facial bones. Injuries to these tissues can have significant functional and aesthetic consequences, impacting speech, chewing, oral health, and facial aesthetics.
Recent studies have explored the potential of MSCs in craniofacial and oral soft tissue regeneration. For instance, Chen et al. (2017) studied calcium phosphate scaffold and endothelial cell co-culture with MSCs from various sources for their respective angiogenic and osteogenic characteristics in vivo and demonstrated that these constructs are promising for craniofacial bone reconstruction application [16]. Srinivasan et al. (2021) demonstrated the in vitro bone regenerative capacity of MSCs from human neural crest stem cells in comparison to bone marrow-derived MSCs for craniofacial bone reconstruction [17]. The embryonic origin of MSCs was related to the greater regenerative capacity as it resulted in enhanced mineralization and differential expression of genes linked to FGF in a 3D polycaprolactone-tricalcium phosphate (PCL-TCP) scaffold system.
Researchers have investigated that the combination of MSC-derived exosomes with scaffolds promotes tissue repair and regeneration in craniofacial applications without cell transplantation [18]. The study concludes that the exosomes from dental pulp-derived mesenchymal stem cells (DP-MSCs) cause osteogenic differentiation and mineralization of bone marrow stromal cells when released in a controlled manner from a biodegradable scaffold and facilitate craniofacial bone reconstruction. Similarly, Liu et al. (2021) demonstrated, in a cell-free approach, that exosomes generated from MSCs had a superior effect on angiogenesis, effectively promoting craniofacial soft tissue regeneration [19]. These data provide a novel strategy to use MSCs in regenerative medicine of oral tissue repair. Hydrogels have also received remarkable attention due to their capacity for stimulating bone reconstruction in craniofacial regeneration [20]. Chu et al. (2021) also utilized moldable gelatin-nanohydroxyapatite cryogel with allogeneic BM-MSCs for craniofacial bone regeneration [21].
2.2. MSCs in Nerve Regeneration
MSCs have shown great potential in promoting nerve regeneration through various mechanisms, making them a promising therapeutic approach. MSCs can differentiate into astrocytes, neurons, and Schwann cell-like cells to support neural regeneration [22][23][24]. In addition to their differentiation capacity, MSCs secrete a variety of neurotrophic factors and growth factors, including brain-derived neurotrophic factor (BDNF), neural growth factor (NGF), and glia cell line-derived neurotropic factor (GDNF), which supports neuron survival and axonal regeneration [25]. The paracrine effects of MSCs on the local microenvironment contribute to immunomodulation, promotion of cell survival, and reduction in inflammation, creating a favorable milieu for nerve regeneration [26][27]. For instance, Chen et al. (2019) demonstrated the superiority of a human MSC-conditioned medium (CM) supplemented with bFGF, EGF, and patient plasma, namely, a neural regeneration laboratory medium (NRLM), on spinal cord injury in both in vitro and in vivo models [28]. The study showed that NRLM-conditioned media were better than standard media in inflammation reduction and neurite regeneration effects in vitro and improved functional restoration in spinal cord injury rats in vivo. Song et al. (2020) also demonstrated the modulatory effects of an MSC-CM and HGF in the presence of bone morphogenetic protein (BMP) 4, with or without a c-Met antibody, on neuronal stem cell differentiation and recovery of spinal cord injury [29]. Recently, Chouaib et al. (2023) also showed that a dental pulp-derived (DP)-MSC-CM can significantly stimulate neurite outgrowth in primary sensory neurons [30]. Chen et al. (2022) employed 3D-printed collagen/silk fibroin scaffolds to carry the secretome derived from UM-MSCs and demonstrated amelioration of neurological dysfunction after implantation of the scaffold in a rat model of spinal cord injury [31]. BM-MSCs and AD-MSCs are currently the most studied cell sources for CNS repair and hold similar neuronal differentiation capacities, but the number of cytokines and growth factors that AD-MSCs produced compared to BM-MSCs was shown to be significantly higher [32][33]. Apart from BM- and AD-MSCs, DP-MSCs have also shown regenerative capacity for nerve repair. Saez et al. (2019) investigated the therapeutic effects of MSCs derived from human dental pulp in a rat model of facial nerve injury. The MSCs promoted nerve regeneration and functional recovery by improving axonal regrowth and modulating the inflammatory response [34]. In short, the interaction between MSCs and nerve stem cells has unfolded a promising area of research in the field of neuronal regeneration [35]. Cui et al. (2022) studied the exosomes derived from UM-MSCs and showed that these exosomes inhibited the activation of microglia and astrocytes during brain injury, thereby promoting functional recovery in rats after traumatic brain injury [36]. Similarly, Li et al. (2022) studied exosomes from lipopolysaccharide-preconditioned BM-MSCs that were able to shift the pro-inflammation macrophage into a pro-regeneration macrophage and hence accelerated peripheral nerve regeneration via M2 macrophage polarization [37].
2.3. MSCs in Blood Vessel Regeneration
Several researchers have investigated the effects of MSCs on angiogenesis and vasculogenesis, the formation of new blood vessels. It is well known that MSCs play a role in vascularization. In vivo studies have demonstrated that MSCs function similarly to perivascular cells [38][39][40][41]. Furthermore, they can differentiate into endothelial cells and form capillary tube-like structures [39][40]. Recently, Jang et al. (2023) described that MSCs cultured with an endothelial cell culture medium and supplemented with vascular endothelial growth factor (VEGF) contribute to vasculogenesis by their sprouting capability in response to bFGF. These MSCs increase the angiogenic sprouting of human umbilical vein endothelial cells by secretion of a paracrine factor called HGF [42].
The ability of MSCs to enhance angiogenesis has significant implications for restoring blood flow and tissue function. In addition to their involvement in blood vessel regeneration, MSCs exert immunomodulatory effects. These effects can indirectly influence the regeneration of blood vessels by regulating the inflammatory response and facilitating tissue repair [43][44]. Nammian et al. (2021) explored the application of allogenic BM-MSCs and AD-MSCs and found that they secrete immunomodulatory cytokines that are pro-angiogenic and lead to the formation of blood vessels [45]. Modulating the immune response is crucial to creating a favorable environment for blood vessel regeneration. MSCs represent a preference for making autologous tissue-engineered vascular grafts, as summarized by Afra et al. (2020) [46]. Apart from allogenic MSCs, autologous injections of AD-MSCs have also been shown to promote blood vessel regeneration [47].
2.4. MSCs in Muscle Regeneration
Muscle tissue regeneration plays a crucial role in restoring the structure and function of injured or degenerated muscles. Skeletal muscle is a tissue that in homeostatic conditions performs regeneration activity with the help of tissue-resident muscle stem cells called satellite cells. These cells play a central role in skeletal muscle regeneration after injury. But, in diseases like Duchenne muscular dystrophy (DMD), these satellite cells accumulate abnormalities and are no longer capable of promoting cell proliferation and tissue regeneration [48]. MSCs have emerged as a promising approach for muscle regeneration due to their myogenic differentiation capacity and paracrine effects. MSCs can differentiate into myocytes and contribute to muscle fiber repair and regeneration, facilitating the restoration of muscle architecture and functionality. Additionally, MSCs secrete a range of growth factors, cytokines, and extracellular matrix components that promote myogenesis and angiogenesis while modulating the local inflammatory response, creating a favorable microenvironment for muscle regeneration and contributing to tissue repair [49][50][51].
Efforts have also been made to check for the effectiveness of just the secretome of MSCs in skeletal muscle regeneration. In a study conducted by Robert Mitchell et al. (2019), the regenerative potential of a cell-free MSC secretome was demonstrated in vivo using a CTX mouse model of acute muscle injury [52]. The whole secretome of an AD-MSC consists of soluble proteins and extracellular vesicles (EVs) containing miRNA and soluble proteins as their cargo. The researchers showed that the AD-MSC secretome is capable of promoting cell proliferation and migration in vivo in the CTX mouse model for muscle injury. Then, they tried to asses the differential capability of secretome and EVs in muscle regeneration, and it was seen that both these fractions increased the cross-sectional area of newly regenerated muscle fibers and reduced the infiltration of macrophages with the EV fraction producing stronger effects. The EV fraction, in addition to stimulating cell proliferation and migration, also reduced the inflammation levels in the muscle injury and regeneration model, as supported by other studies [53][54]. Furthermore, several studies observed that MSCs improved muscle regeneration in volumetric muscle loss (VML), increased angiogenesis, and enhanced functional recovery [55]. These findings further support the beneficial effects of MSCs in muscle regeneration.
2.5. MSCs in Fibrous Tissue Regeneration
Fibrous tissues, such as tendons and ligaments, are susceptible to injury and often have limited regenerative capacity. MSCs have shown promise in fibrous tissue regeneration due to their ability to differentiate into tenocytes or fibroblasts and their paracrine effects that promote tissue healing and remodeling. MSCs can contribute to the regeneration of fibrous tissues by directly differentiating into tenocytes or fibroblasts, thereby enhancing the synthesis of extracellular matrix components and restoring tissue structure and function. Additionally, MSCs secrete a range of growth factors and cytokines that modulate the inflammatory response, promote angiogenesis, and stimulate endogenous repair mechanisms.
In vitro studies have provided valuable insights into the potential of MSCs for fibrous tissue regeneration. For example, Yang et al. (2013) conducted in vitro experiments using AD-MSCs and demonstrated their ability to differentiate, in the presence of a tendon ECM, into fibroblast-like cells and promote the secretion of collagen and other extracellular matrix components, indicating their potential in ligament regeneration [56].
In contrast, studies have also used different pro-tenogenic factors to induce tenogenesis. An in vitro study has shown that equine AD-MSCs differentiate into tenocytes in response to the combination of platelet-derived growth factor-BB (PDGF-BB) and GDF-6 [57]. Another in vitro study has shown the promising role of GDF-7 for the regeneration of the tendon–bone interface due to its ability to differentiate into multiple lineages [58].
3. Conclusions
In conclusion, MSCs hold tremendous promise for tissue regeneration and repair due to their unique properties, including their ease of isolation and in vitro maintenance, multipotency, immunomodulatory effect, and paracrine activity. MSCs have shown great potential in various fields of regenerative medicine, as discussed in the article, such as oral and craniofacial tissue reconstruction, neuronal regeneration, as well as blood vessels, muscles, and fibrous tissue regeneration. The application strategies are numerous such as supplementation of growth factors, delivery with biodegradable scaffolds, and utilization of MSC-derived soluble molecules.
MSCs provide several benefits, but there are still a lot of obstacles to overcome. Understanding how the tissue environment affects the destiny and capabilities of MSCs is crucial in terms of lineage differentiation. The clinical transformation of MSCs is surely hampered by the difficult-to-unify differentiation potential, surface markers, and transcription of various tissue-derived MSCs. The distinct immunomodulatory characteristics of MSCs are vital for their roles, although it is yet unclear how the immune system in the context of MSCs is regulated. Moreover, to develop effective and secure regenerative medicine applications, it is also crucial to comprehend the paracrine route involved in the healing process controlled by MSCs.
This entry is adapted from the peer-reviewed paper 10.3390/medicina59081449
References
- Miller, L.W. New strategies to enhance stem cell homing for tissue repair. In Stem Cell and Gene Therapy for Cardiovascular Disease; Academic Press: Cambridge, MA, USA, 2016; pp. 485–496.
- Krafts, K.P. Tissue repair: The hidden drama. Organogenesis 2010, 6, 225–233.
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84.
- Bolton, L. Diabetic foot ulcer: Treatment challenges. Wounds 2022, 34, 175–177.
- Gibbs, S.; Roffel, S.; Meyer, M.; Gasser, A. Biology of soft tissue repair: Gingival epithelium in wound healing and attachment to the tooth and abutment surface. Eur. Cells Mater. 2019, 38, 63–78.
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.-L.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25.
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2017, 23, 83–99.
- Yan, R.; Cigliola, V.; Oonk, K.A.; Petrover, Z.; DeLuca, S.; Wolfson, D.W.; Vekstein, A.; Mendiola, M.A.; Devlin, G.; Bishawi, M.; et al. An enhancer-based gene-therapy strategy for spatiotemporal control of cargoes during tissue repair. Cell Stem Cell 2023, 30, 96–111.e6.
- Gentile, P.; Garcovich, S. Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma, and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. Int. J. Mol. Sci. 2021, 22, 1538.
- Klingemann, H.; Matzilevich, D.; Marchand, J. Mesenchymal Stem Cells—Sources and Clinical Applications. Transfus. Med. Hemother. 2008, 35, 272–277.
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886.
- Stanko, P.; Kaiserova, K.; Altanerova, V.; Altaner, C. Comparison of human mesenchymal stem cells derived from dental pulp, bone marrow, adipose tissue, and umbilical cord tissue by gene expression. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2014, 158, 373–377.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- Kelly, K.; Rasko, J.E.J. Mesenchymal Stromal Cells for the Treatment of Graft Versus Host Disease. Front. Immunol. 2021, 12, 761616.
- Borrelli, M.R.; Hu, M.S.; Longaker, M.T.; Lorenz, H.P. Tissue Engineering and Regenerative Medicine in Craniofacial Reconstruction and Facial Aesthetics. J. Craniofac Surg. 2020, 31, 15–27.
- Chen, W.; Liu, X.; Chen, Q.; Bao, C.; Zhao, L.; Zhu, Z.; Xu, H.H. Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell co-culture with human bone marrow mesenchymal stem cells (MSCs), human umbilical cord MSCs, human induced pluripotent stem cell-derived MSCs and human embryonic stem cell-derived MSCs. J. Tissue Eng. Regen. Med. 2018, 12, 191–203.
- Srinivasan, A.; Teo, N.; Poon, K.J.; Tiwari, P.; Ravichandran, A.; Wen, F.; Teoh, S.H.; Lim, T.C.; Toh, Y.C. Comparative craniofacial bone regeneration capacities of mesenchymal stem cells derived from human neural crest stem cells and bone marrow. ACS Biomater. Sci. Eng. 2020, 7, 207–221.
- Swanson, W.B.; Zhang, Z.; Xiu, K.; Gong, T.; Eberle, M.; Wang, Z.; Ma, P.X. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020, 118, 215–232.
- Liu, Y.; Zhuang, X.; Yu, S.; Yang, N.; Zeng, J.; Liu, X.; Chen, X. Exosomes derived from stem cells from apical papilla promote craniofacial soft tissue regeneration by enhancing Cdc42-mediated vascularization. Stem Cell Res. Ther. 2021, 12, 76.
- Liu, X.; Fang, T.; Shi, T.; Wang, Y.; Liu, G. Hydrogels provide microenvironments to mesenchymal stem cells for craniofacial bone regeneration. J. Biomater. Appl. 2023, 38, 3–24.
- Chu, C.-F.; Mao, S.-H.; Shyu, V.B.-H.; Chen, C.-H.; Chen, C.-T. Allogeneic Bone-Marrow Mesenchymal Stem Cell with Moldable Cryogel for Craniofacial Bone Regeneration. J. Pers. Med. 2021, 11, 1326.
- Dezawa, M.; Takahashi, I.; Esaki, M.; Takano, M.; Sawada, H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur. J. Neurosci. 2001, 14, 1771–1776.
- Ramli, K.; Aminath Gasim, I.; Ahmad, A.A.; Hassan, S.; Law, Z.K.; Tan, G.C.; Baharuddin, A.; Naicker, A.S.; Htwe, O.; Mohammed Haflah, N.H.; et al. Human bone marrow-derived MSCs spontaneously express specific Schwann cell markers. Cell Biol. Int. 2019, 43, 233–252.
- Zhang, P.; He, X.; Liu, K.; Zhao, F.; Fu, Z.; Zhang, D.; Zhang, Q.; Jiang, B. Bone Marrow Stromal Cells Differentiated into Functional Schwann Cells in Injured Rats Sciatic Nerve. Artif. Cells Blood Substit. Biotechnol. 2004, 32, 509–518.
- Paradisi, M.; Alviano, F.; Pirondi, S.; Lanzoni, G.; Fernandez, M.; Lizzo, G.; Giardino, L.; Giuliani, A.; Costa, R.; Marchionni, C.; et al. Human Mesenchymal Stem Cells Produce Bioactive Neurotrophic Factors: Source, Individual Variability and Differentiation Issues. Int. J. Immunopathol. Pharmacol. 2014, 27, 391–402.
- Li, X.; Guan, Y.; Li, C.; Zhang, T.; Meng, F.; Zhang, J.; Li, J.; Chen, S.; Wang, Q.; Wang, Y.; et al. Immunomodulatory effects of mesenchymal stem cells in peripheral nerve injury. Stem Cell Res. Ther. 2022, 13, 18.
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2021, 11, 591065.
- Chen, Y.-T.; Tsai, M.-J.; Hsieh, N.; Lo, M.-J.; Lee, M.-J.; Cheng, H.; Huang, W.C. The superiority of conditioned medium derived from rapidly expanded mesenchymal stem cells for neural repair. Stem Cell Res. Ther. 2019, 10, 390.
- Song, P.; Han, T.; Xiang, X.; Wang, Y.; Fang, H.; Niu, Y.; Shen, C. The role of hepatocyte growth factor in mesenchymal stem cell-induced recovery in spinal cord injured rats. Stem Cell Res. Ther. 2020, 11, 178.
- Chouaib, B.; Collart-Dutilleul, P.-Y.; Blanc-Sylvestre, N.; Younes, R.; Gergely, C.; Raoul, C.; Scamps, F.; Cuisinier, F.; Romieu, O. Identification of secreted factors in dental pulp cell-conditioned medium optimized for neuronal growth. Neurochem. Int. 2021, 144, 104961.
- Chen, C.; Xu, H.-H.; Liu, X.-Y.; Zhang, Y.-S.; Zhong, L.; Wang, Y.-W.; Xu, L.; Wei, P.; Chen, Y.X.; Liu, P.; et al. 3D printed collagen/silk fibroin scaffolds carrying the secretome of human umbilical mesenchymal stem cells ameliorated neurological dysfunction after spinal cord injury in rats. Regen. Biomater. 2022, 9, rbac014.
- Zhou, Z.; Chen, Y.; Zhang, H.; Min, S.; Yu, B.; He, B.; Jin, A. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy 2013, 15, 434–448.
- Chung, C.-S.; Fujita, N.; Kawahara, N.; Yui, S.; Nam, E.; Nishimura, R. A comparison of neurosphere differentiation potential of canine bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells. J. Vet. Med. Sci. 2013, 75, 879–886.
- Saez, D.M.; Sasaki, R.T.; de Martins, D.O.; Chacur, M.; Kerkis, I.; da Silva, M.C.P. Rat Facial Nerve Regeneration with Human Immature Dental Pulp Stem Cells. Cell Transpl. 2019, 28, 1573–1584.
- Kaminska, A.; Radoszkiewicz, K.; Rybkowska, P.; Wedzinska, A.; Sarnowska, A. Interaction of neural stem cells (NSCs) and mesenchymal stem cells (MSCs) as a promising approach in brain study and nerve regeneration. Cells 2022, 11, 1464.
- Cui, L.; Luo, W.; Jiang, W.; Li, H.; Xu, J.; Liu, X.; Wang, B.; Wang, J.; Chen, G. Human umbilical cord mesenchymal stem cell-derived exosomes promote neurological function recovery in rat after traumatic brain injury by inhibiting the activation of microglia and astrocyte. Regen. Ther. 2022, 21, 282–287.
- Li, C.; Li, X.; Shi, Z.; Wu, P.; Fu, J.; Tang, J.; Qing, L. Exosomes from LPS-preconditioned bone marrow MSCs accelerated peripheral nerve regeneration via M2 macrophage polarization: Involvement of TSG-6/NF-κB/NLRP3 signaling pathway. Exp. Neurol. 2022, 356, 114139.
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313.
- Oswald, J.; Boxberger, S.; Jørgensen, B.; Feldmann, S.; Ehninger, G.; Bornhäuser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384.
- Du, W.J.; Chi, Y.; Yang, Z.X.; Li, Z.J.; Cui, J.J.; Song, B.Q.; Li, X.; Yang, S.G.; Han, Z.B.; Han, Z.C. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res. Ther. 2016, 7, 163.
- Shi, S.; Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 2003, 18, 696–704.
- Jang, H.H.; Son, Y.; Park, G.; Park, K.-S. Bone Marrow-Derived Vasculogenic Mesenchymal Stem Cells Enhance In Vitro Angiogenic Sprouting of Human Umbilical Vein Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 413.
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 01191.
- Saldaña, L.; Bensiamar, F.; Vallés, G.; Mancebo, F.J.; García-Rey, E.; Vilaboa, N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res. Ther. 2019, 10, 58.
- Nammian, P.; Asadi-Yousefabad, S.-L.; Daneshi, S.; Sheikhha, M.H.; Tabei, S.M.B.; Razban, V. Comparative analysis of mouse bone marrow and adipose tissue mesenchymal stem cells for critical limb ischemia cell therapy. Stem Cell Res. Ther. 2021, 12, 58.
- Afra, S.; Matin, M.M. Potential of mesenchymal stem cells for bioengineered blood vessels in comparison with other eligible cell sources. Cell Tissue Res. 2020, 380, 1–13.
- Zhou, X.; Ning, K.; Ling, B.; Chen, X.; Cheng, H.; Lu, B.; Gao, Z.; Xu, J. Multiple Injections of Autologous Adipose-Derived Stem Cells Accelerate the Burn Wound Healing Process and Promote Blood Vessel Regeneration in a Rat Model. Stem Cells Dev. 2019, 28, 1463–1472.
- Dumont, N.A.; Wang, Y.X.; Von Maltzahn, J.; Pasut, A.; Bentzinger, C.F.; Brun, C.E.; Rudnicki, M.A. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med. 2015, 21, 1455–1463.
- Dörnen, J.; Dittmar, T. The Role of MSCs and Cell Fusion in Tissue Regeneration. Int. J. Mol. Sci. 2021, 22, 980.
- Gorecka, A.; Salemi, S.; Haralampieva, D.; Moalli, F.; Stroka, D.; Candinas, D.; Eberli, D.; Brügger, L. Autologous transplantation of adipose-derived stem cells improves functional recovery of skeletal muscle without direct participation in new myofiber formation. Stem Cell Res. Ther. 2018, 9, 195.
- Ichiseki, T.; Shimasaki, M.; Ueda, S.; Hirata, H.; Souma, D.; Kawahara, N.; Ueda, Y. Efficacy of Rectal Systemic Administration of Mesenchymal Stem Cells to Injury Sites via the CXCL12/CXCR4 Axis to Promote Regeneration in a Rabbit Skeletal Muscle Injury Model. Cells 2023, 12, 1729.
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.T.; Tomkins, J.E.; Denecke, B.; Musante, L.; et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res. Ther. 2019, 10, 116.
- Khan, I.; Ali, A.; Akhter, M.A.; Naeem, N.; Chotani, M.A.; Iqbal, H.; Kabir, N.; Atiq, M.; Salim, A. Epac-Rap1-activated mesenchymal stem cells improve cardiac function in rat model of myocardial infarction. Cardiovasc. Ther. 2017, 35, e12248.
- Chen, Y.; Shen, H.; Ding, Y.; Yu, Y.; Shao, L.; Shen, Z. The application of umbilical cord-derived MSCs in cardiovascular diseases. J. Cell. Mol. Med. 2021, 25, 8103–8114.
- Shayan, M.; Huang, N.F. Pre-Clinical Cell Therapeutic Approaches for Repair of Volumetric Muscle Loss. Bioengineering 2020, 7, 97.
- Yang, G.; Rothrauff, B.B.; Lin, H.; Gottardi, R.; Alexander, P.G.; Tuan, R.S. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials 2013, 34, 9295–9306.
- Javanshir, S.; Younesi Soltani, F.; Dowlati, G.; Parham, A.; Naderi-Meshkin, H. Induction of tenogenic differentiation of equine adipose-derived mesenchymal stem cells by platelet-derived growth factor-BB and growth differentiation factor-6. Mol. Biol. Rep. 2020, 47, 6855–6862.
- Kumlin, M.; Lindberg, K.; Haldosen, L.-A.; Felländer-Tsai, L.; Li, Y. Growth Differentiation Factor 7 promotes multiple-lineage differentiation in tenogenic cultures of mesenchymal stem cells. Injury 2022, 53, 4165–4168.
This entry is offline, you can click here to edit this entry!
