Drug and substance misuse refers to the usage of substances for purposes that are illegal or against medical advice. It has negative consequences for health and can manifest as drug dependence or as one of a variety of other problematic or destructive behaviors. This behavior can result in drug dependence and various social and mental health difficulties. The consequences of substance misuse can be severe, including car accidents, driving under the influence arrests, Domestic violence, sexual harassment, child neglect and abuse, suicide attempts and fatalities, strokes, and overdose deaths.
1. Cannabis
Based on the pharmacological effects, ∆9-THC has neurobehavioral effects mediated by the activation of cannabinoid receptor type 1 (CB1) in the Central Nervous System (CNS). A G protein-linked receptor, CB1, plays a role in several physiological processes, such as appetite, pain perception, mood, and memory
[1]. Observational studies indicated that recreational cannabis use may lead to acute poisonings, neurotoxicity, and dangerous outcomes, particularly for children accidentally consuming homemade edibles or concentrated products at home, resulting in intensive care unit admissions and even death, while also being associated with an acceleration of cardiovascular age, irrespective of other factors, suggesting potential adverse effects on the cardiovascular system
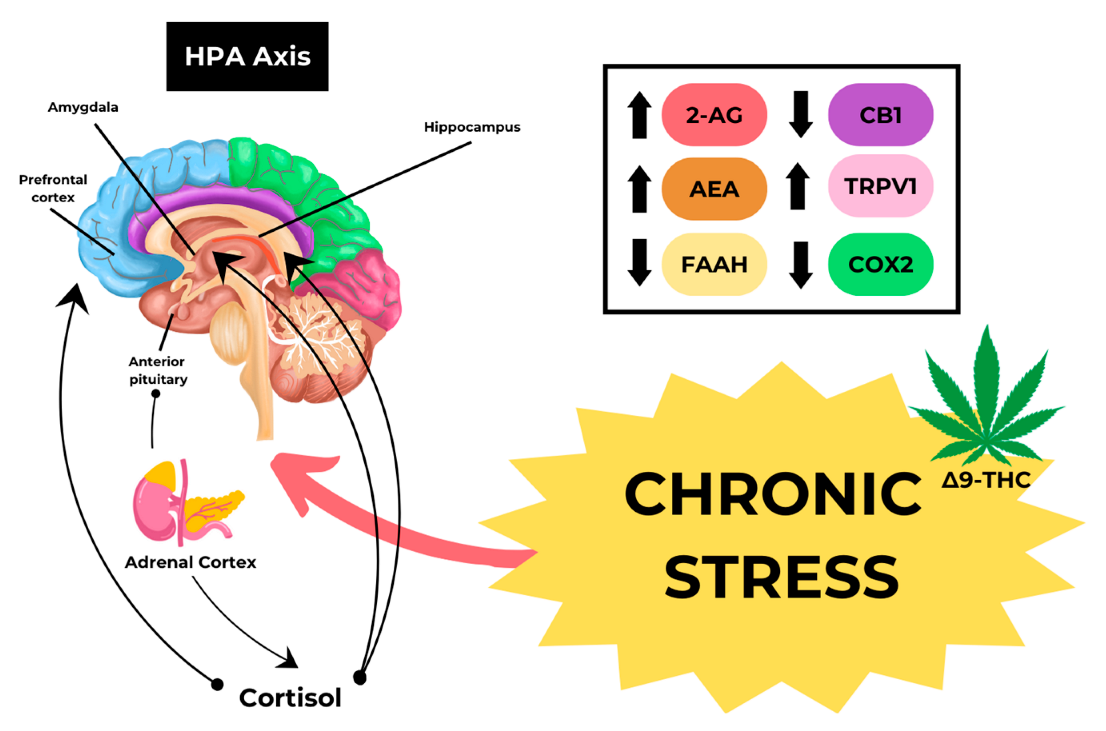
[2][3][4]. One of the fatal consequences of endocannabinoid system dysregulation is Cannabinoid Hyperemesis Syndrome (CHS). The exact pathophysiological mechanism of CHS is not yet fully understood; however, chronic daily cannabis use has the potential to desensitize or suppress CB1 receptors, which would render cannabinoids ineffective as an antiemetic
[5][6]. The development of CHS may be associated with an increase in Transient Receptor Potential Vanilloid 1 (TRPV1) due to chronic stress, as shown in
Figure 1.
Figure 1. Mechanism of CHS caused by Cannabis.
The primary route of THC delivery is through smoking, accounting for approximately 20% to 70% of THC intake. Smoking a cannabis cigarette containing 500 to 1000 mg of cannabis can deliver a THC dose of 0.2–4.4 mg, while the pharmacological effects of cannabis typically require a dose of 2–22 mg. THC concentrations in the brain generally represent only ∼1% of the administered dose and are typically equivalent to 2–44 μg
[7]. Doses > 7.5 mg/m
2 inhaled in adults have been reported to cause more severe symptoms, such as hypotension, respiratory depression, and ataxia. Long-term cannabis consumption can result in cyclic hyperemesis, behavioral problems, and bronchospasm due to Inhalation
[8]. Although there is no specific data on the toxic concentration of THC in humans, high doses of cannabis consumption are known to cause organ damage.
2. Amphetamine-Type Stimulant
Amphetamine-Type Stimulant (ATS) is a class of drugs regulated by the 1971 Convention on Psychotropic Substances. It is made up of synthetic stimulants. This group includes amphetamine, methamphetamine, 3,4-methylenedioxy-methylamphetamine (MDMA and ecstasy), and its analogs. Methamphetamine (MA) is the second most abused illegal drug in the world, following cannabis, and its user population almost surpasses the combined number of heroin and cocaine users. East and Southeast Asia have the highest number of MA users, accounting for about two-thirds of the global user population, followed by the Americas, particularly the United States and Northern Mexico, where approximately one-fifth of MA users reside. In 2020, 34.080 million people used MA globally, representing 0.68% of the total global population. In Europe, MA is the second most commonly used stimulant after cocaine
[9].
The main administration routes of MA overexposure are intravenous injection, intranasal insufflation, smoking, and ingestion. Another route that has been reported is the transrectal route, one of the cases described by Takasu, et al. (2021)
[10]. The case explained that a deceased individual with a blood MA level of 9.44 μg/mL was considered fatal. In another case, McIntyre et al. (2013) reported methamphetamine levels of 13 mg/L in peripheral blood, leading to pulmonary edema and congestion. No other cause of death was identified, and acute MA intoxication was determined to cause death
[11]. Various studies have reported the lethal blood MA level, which according to the Winek criteria, is >10 μg/mL
[12]. The lethal MA blood level can range from 1.4 to 13 μg/mL
[13]. The reported therapeutic doses, according to studies, are 0.062–0.291 mg/L (30 mg oral dose) and 0.132 mg/L (0.50 mg/kg single intravenous dose)
[11]. MA activates the neurotransmitter system, which increases body temperature, blood pressure, pulse rate, cutaneous vasoconstriction, and respiratory rate. High doses of MA continue to be abused, resulting in severe neurotoxicity, cardiovascular dysfunction, pulmonary disease, and several other diseases
[14].
3. Opioids
Opioids are a class of drugs used for reducing severe pain. This class includes both illegal drugs such as heroin and synthetic opioids such as fentanyl, as well as prescription pain relievers such as codeine, morphine, and tramadol, among others. According to data compiled by UNODC, in 2020, approximately 61,290 million individuals worldwide, representing 1.2% of the global population, consumed opioids. Half of the opioid users reside in South Asia and Southwest Asia. Opioids are considered the most lethal drug group and are responsible for two-thirds of deaths directly related to drugs, primarily due to overdoses
[15].
There are 3 types of opioid receptors, namely µ (MOP), δ (DOP), and κ (KOP). Among these receptors, MOP receptors are the primary target for severe pain relief in opioid therapy
[16]. Fentanyl is a synthetic opioid with a strong affinity for MOP, making it a strong analgesic about 50 to 100 times stronger than morphine. Fentanyl is well-known in the form of a transdermal patch which is very comfortable for patients with acute and chronic pain
[17]. Fentanyl poisoning can lead to Opioid-Induced Respiratory Depression (OIRD), characterized by a significant decrease in respiratory frequency and regularity, originating from the medullary preBötzinger complex (preBötC)
[18][19]. A case reported by Karen L. Woodall et al. described the death of a 42-year-old male who was found with evidence of drug paraphernalia and numerous prescription bottles. Postmortem examination revealed pieces of plastic from a fentanyl patch in his mouth and posterior oropharynx, but not obstructing the airway. Toxicological analysis indicated a high fentanyl concentration in his heart blood, along with the presence of other drugs. The cause of death was attributed to a fentanyl overdose
[20].
Tramadol, or O-desmethyl-tramadol (its active metabolite), is an opioid with a high affinity for MOP. It reduces pain by preventing serotonin and norepinephrine from reuptaking their respective amounts in the brain. The therapeutic dose range for a single dose of tramadol is typically 50–100 mg every 4–6 h, with a maximum recommended dose of 400 mg/day. Tramadol toxicity can affect various organ systems, including the CNS (causing seizures, CNS depression, and low-grade coma), the cardiovascular system (resulting in symptoms ranging from palpitations to life-threatening complications such as cardiopulmonary arrest), and may lead to conditions such as rhabdomyolysis and serotonin syndrome
[21][22]. A case reported by Koen De Decker et al. mentioned the death of a 28-year-old Caucasian man who had been treated with tramadol for vague abdominal complaints. The patient ingested a benzodiazepine and snored heavily at night. He experienced apnea and cardiac arrest in the morning, leading to extreme acidosis and hypoglycemia. Despite aggressive resuscitation efforts and supportive therapies, he developed acute hepatic and renal failure, with a liver biopsy revealing steatosis and centrolobular necrosis. Clinical toxicological screening ruled out the presence of other toxic drugs, and GC-MS analysis showed only the presence of tramadol
[23].
Morphine is a classic opioid class that acts primarily on MOP receptors within the CNS and the Peripheral Nervous System (PNS). Common side effects that occur when consuming morphine are CNS depression, nausea, vomiting, and urinary retention. A morphine overdose can lead to the accumulation of morphine in the brain, resulting in respiratory depression and death
[24]. A case reported by Shepard Siegel and Delbert W. Ellsworth showed a patient who, unfortunately, passed away while receiving morphine injections for an extended period due to a severe medical condition. The morphine injections were given four times a day for four weeks, with a gradual increase in dosage. The patient’s environment was typically dimly lit with hospital-type apparatus, where the morphine injections were administered without issues. However, on the day of the overdose, the patient received the injection in a brightly lit living room, which led to an atypical reaction, including small pupils and shallow breathing. The physician suspected a morphine overdose based on these symptoms, and the patient eventually passed away
[25].
4. Cocaine
In 2020, it is estimated that approximately 21.5 million individuals, accounting for 0.4% of the world’s population between the ages of 15 and 64, used cocaine at least once in the previous year. Although the prevalence of cocaine use has slightly increased since 2010, the actual number of cocaine users has grown by 32% due to population growth
[9]. Misuse of cocaine poses various potentially fatal side effects, including acute myocardial. Ventricular free-wall rupture, a highly lethal complication, occurs in up to 2% of patients facing acute myocardial infarction
[26][27].
5. New Psychoactive Substances
The term “New Psychoactive Substances” (NPS) refers to compounds of abuse, whether in their pure form as well as in preparations, which may be analogs of illegal substances or newly developed chemicals intended to imitate the psychoactive effects of prohibited substances
[28]. NPS can be categorized into 4 primary categories: synthetic stimulants, synthetic cannabinoids, synthetic hallucinogens, and synthetic depressants. Mephedrone and N-ethylpentylone, belong to the class of synthetic cathinone, indicating the frequent inappropriate use of synthetic stimulants
[29]. In 2020, data from 77 nations across all continents indicated the use of NPS within their territories. Among the mentioned NPS, ketamine was reported by 56 nations, and 38 countries mentioned synthetic cannabinoids. Of the 23 nations with available data, 21 stated that 1% or less of their population had used NPS in the previous year. Due to their relatively uncommon usage, NPS is not regulated by the Convention on Psychotropic Substances of 1971 or the Single Convention on Narcotic Drugs of 1961, raising public health concerns
[9]. Mephedrone has a similar abuse potential characteristic to MDMA, with a few differences, such as a quicker onset and less long duration of effects, likely due to its rapid elimination half-life
[30]. N-ethylpentylone, a psychomotor stimulant, may have serious negative effects to cause severe cardiovascular and neurological adverse effects. The lethal dose (LD50) of N-ethylpentylone in mice was reported as 240 mg/kg, but the route of administration was not specified
[31]. Seizures, muscle spasms, hallucinations, hyperthermia, nausea, vomiting, rhabdomyolysis, renal failure, and arrhythmia are severe side effects linked to the usage of NPS.
6. Hallucinogens
Hallucinogens are a diverse group of naturally occurring and synthetic drugs that induce distorted states of consciousness, perception, thinking, and feeling, accompanied by different degrees of auditory or visual hallucinations. The two primary categories of hallucinogens are psychedelic and dissociative. Psychedelic substances, such as psilocybin and lysergic acid diethylamide (LSD), primarily affect 5-hydroxy-tryptamine (5-HT)2A receptors as the target for serotonin. Meanwhile, dissociative drugs work by blocking the action of N-methyl-D-aspartate (NMDA) receptors. Member States implies that hallucinogenic drug use is ranked lower than other substances, which suggests that it is less of a worry than using cannabis and other drugs globally, with an average score of 5.3 throughout 2013–2017
[15].
7. Safety and Toxicological Evaluation
Use and misuse of alcohol, nicotine, and illicit drugs, and misuse of prescription drugs cost Americans more than $700 billion a year in increased health care costs, crime, and lost productivity
[32]. The misuse of drugs will cause addiction as a chronic, relapsing disorder characterized by compulsive drug seeking. It is considered a brain disorder because it involves functional changes to brain circuits involved in reward, stress, and self-control
[33]. Deaths involving synthetic opioids other than methadone (primarily fentanyl) continued to rise, with 70,601 overdose deaths reported. Those involving stimulants, including cocaine or psychostimulants with abuse potential (primarily methamphetamine), continued to increase, with 32,537 overdose deaths in 2021
[34].
Since most patients who overdose on drugs are lethargic or comatose, the history is usually obtained from family, friends, bystanders, and emergency medical service providers. On many occasions at the scene, one may find pills, empty bottles, needles, syringes, and other drug paraphernalia. Other features that one should try and obtain in the history are the amount of drug ingested, congestion, and time of ingestion
[35]. Rapid identification of the toxidrome saves time in evaluating and managing a poisoned patient. (
Figure 2).
Figure 2. Toxidrome Approach to Poisoning Due to Drugs of Abuse. Created with
www.biorender.com, accessed on 10 August 2023.
Substance Abuse Evaluations (SAEs) are conducted by medical professionals trained to evaluate individuals suspected of abusing substances. These evaluations include physical examinations, psychological testing, and interviews with family members, friends, coworkers, supervisors, and others familiar with the person being evaluated. The evaluation results are then used to determine whether or not the individual should be referred for treatment. SAEs can also determine the duration to which substance use has impacted their life and the degree of an individual’s drug or alcohol abuse/addiction if there are co-occurring concerns (e.g., depression, anxiety, etc.)
[36].
This entry is adapted from the peer-reviewed paper 10.3390/toxics11090756