In fungi, the methylcitrate cycle converts cytotoxic propionyl-coenzyme A (CoA) to pyruvate, which enters gluconeogenesis. The glyoxylate cycle converts acetyl-CoA to succinate, which enters gluconeogenesis. The tricarboxylic acid cycle is a central carbon metabolic pathway that connects the methylcitrate cycle, the glyoxylate cycle, and other metabolisms for lipids, carbohydrates, and amino acids. Fungal citrate synthase and 2-methylcitrate synthase as well as isocitrate lyase and 2-methylisocitrate lyase, each evolved from a common ancestral protein. Impairment of the methylcitrate cycle leads to the accumulation of toxic intermediates such as propionyl-CoA, 2-methylcitrate, and 2-methylisocitrate in fungal cells, which in turn inhibits the activity of many enzymes such as dehydrogenases and remodels cellular carbon metabolic processes. The methylcitrate cycle and the glyoxylate cycle synergistically regulate carbon source utilization as well as fungal growth, development, and pathogenic process in pathogenic fungi.
1. Introduction
Propionyl-CoA is an intermediate metabolite produced by organisms during metabolism, which is toxic to cells [
1]. Propionate, amino acids (isoleucine, methionine, threonine, and valine), thymine, and odd chain fatty acids are catabolized to yield propionyl-CoA [
2,
3]. Propionate is the second most abundant organic acid naturally occurring in soil. Propionate inhibits the growth of microorganisms and is used as a common food preservative [
4]. Four amino acids (isoleucine, methionine, threonine, and valine) account for about 15% of amino acid abundance in proteins of various environmental microorganisms [
5]. Propionyl-CoA is also produced by cholesterol via side chain oxidation. After propionyl-CoA is produced, organisms have three pathways to catabolize propionyl-CoA. In animals and some bacteria, the methylmalonyl-CoA pathway is a pathway that metabolizes propionyl-CoA [
3]. Propionyl-CoA is sequentially converted to methylmalonyl-CoA, succinyl-CoA, and malate, which is then metabolized to acetyl-CoA and glyoxylate [
6]. Another propionyl-CoA metabolic pathway is the methylcitrate cycle present in fungi and some bacteria [
7,
8]. In the pathogenic fungus
Candida albicans, Otzen et al. proposed a third propionyl-CoA metabolic pathway that propionyl-CoA is metabolized via a modified β-oxidation pathway [
9]. In this β-oxidation pathway, propionyl-CoA is sequentially converted to acrylyl-CoA, 3-hydroxypropionyl-CoA, 3-hydroxypropionate, and malonate semialdehyde, which is then metabolized to acetyl-CoA or acetate [
9].
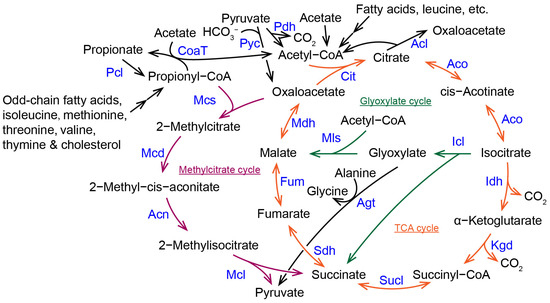
Fungi metabolize acetyl-CoA through the methylcitrate cycle. The methylcitrate cycle shares several metabolic steps with the tricarboxylic acid (TCA) cycle and the glyoxylate pathway (
Figure 1). The TCA cycle is the central pathway of carbon metabolism in all organisms. The glyoxylate cycle is the link between lipid and ketogenic amino acid catabolism and gluconeogenesis pathways in fungi and plants. In the methylcitrate cycle, 2-methylcitrate synthase (Mcs) catalyzes propionyl-CoA and oxaloacetate to produce 2-methylcitrate. Then, 2-methylcitrate is converted to 2-methyl-cis-aconitate and 2-methylisocitrate sequentially by 2-methylcitrate dehydratase (Mcd) and aconitase (Acn). Next, 2-methylisocitrate lyase (Mcl) catalyzes 2-methylisocitrate to cleave into pyruvate and succinate [
7]. Pyruvate and succinate then enter the TCA cycle, gluconeogenesis and other metabolic pathways [
10]. In some bacteria, propionyl-CoA is also metabolized by the methylcitrate cycle [
11].
Figure 1. The methylcitrate cycle and its linkage with the TCA and glyoxylate cycles in fungi. Acl, ATP citrate lyase; Acn, aconitase; Aco, aconitase; Agt, alanine, glyoxylate aminotransferase; Cit, citrate synthase; CoaT, CoA-transferase; Fum, fumarase; Icl, isocitrate lyase; Idh, isocitrate dehydrogenase; Kgd, α-ketoglutarate dehydrogenase; Mcd, 2-methylcitrate dehydratase; Mcl, 2-methylisocitrate lyase; Mcs, 2-methylcitrate synthase; Mls, malate synthase; Mdh, malate dehydrogenase; Pcl, propionate-CoA ligase; Pdh, pyruvate dehydrogenase; Pyc, Pyruvate carboxylase; Sdh, succinate dehydrogenase; Sucl, Succinyl-CoA ligase (succinyl-CoA synthetase).
2. Carbon and Nitrogen Source Utilization and Mycelial Growth
The methylcitrate cycle is an important pathway of carbon metabolism in organisms. Interruption of the methylcitrate cycle leads to the accumulation of intermediate metabolites such as propionyl-CoA in 2-methylcitrate synthase-deficient mutants, 2-methylcitrate in 2-methylcitrate dehydratase-deficient mutants, and 2-methylisocitrate in 2-methylisocitrate lyase-deficient mutants, which are cytotoxic to cells. Excessive accumulation of these products will inhibit the activity of various dehydrogenases in cells, thereby inhibiting cell growth [
1,
12]. The growth of the methylcitrate cycle-deficient mutants is severely inhibited in carbon and nitrogen sources that are metabolized to produce propionyl-CoA directly. Knock-out mutants of the gene encoding 2-methylcitrate synthase, such as Δ
Momcs1 of
Magnaporthe oryzae [
13], Δ
AfmcsA of
Aspergillus fumigatus [
14], and Δ
AnmcsA of
Aspergillus nidulans [
10], failed to grow on media with propionate as the sole carbon source. A 2-methylcitrate dehydratase encoding gene deletion mutant, Δ
Tmmcd of
Talaromyces marneffei [
15], and knock-out mutants of 2-methylisocitrate lyase encoding genes, including Δ
Momcl1 of
M. oryzae [
13], Δ
Gzmcl1 of
Gibberella zeae [
16], Δ
AnmclA of
A. nidulans [
17], and Δ
Tamcl of
Trichoderma atroviride (a biocontrol fungus) [
18], were also unable to grow on media with propionate as the sole carbon source. Valerate, isoleucine, threonine, valine, methionine, or cholesterol are metabolized to produce propionyl-CoA. Δ
Tmmcd grew slowly on media using valerate, valine, methionine, isoleucine, or cholesterol as the carbon source [
15]. Δ
AfmcsA colony growth was inhibited when valine, isoleucine, or methionine was used as the nitrogen source [
14]. Δ
Momcl1 did not grow or grew very slowly on media using threonine, isoleucine, valine or methionine as the sole amino acids [
13]. Growth of Δ
Momcs1 was also slowed on media using isoleucine, valine, or methionine as the sole nitrogen source [
13]. In glycerol, glucose, or acetate media, the addition of propionate inhibited the growth of Δ
AnmcsA, Δ
AnmclA, and Δ
AfmcsA more severely than the wild type [
10,
14,
17].
Growth of most methylcitrate cycle-deficient mutants is also inhibited in media using carbon and nitrogen sources that did not directly produce propionyl-CoA. Mutants Δ
Momcs1, Δ
Momcl1, Δ
Tamcl, and Δ
Gzmcl1 grew slowly when glucose was used as the carbon source [
13,
16,
18]. Δ
Momcl1 grew slowly on media using glutamic acid (not producing propionyl-CoA) or inorganic nitrogen NaNO
3 as a nitrogen source [
13]. Δ
Tamcl also grew slowly on PDA medium or media with acetate and ethanol (C2), pyruvate (C3), butyrate (C4), citrate (C6), Tween 20 (C58), N-acetylglucosamine (NAG), or chitin as the sole carbon source [
18]. However, Δ
Gzmcl1 grew normally in acetate, Tween 60, and linoleic acid media [
16]. Δ
Momcl1 also grew normally when olive oil was the sole carbon source [
13]. This is because the glucose metabolism, lipid metabolism, amino acid metabolism and nucleotide metabolism in cells will normally produce endogenous propionyl-CoA. However, the phenotypes of the methylcitrate cycle-deficient mutants in different fungal strains are diverse, which is related to the different types and amounts of intracellular accumulated intermediates.
Within the same fungal strain, the phenotypes caused by the deletion of different genes of the methylcitrate cycle are diverse, which is also related to the type and quantity of the intermediate compounds accumulated in the mutants. The functions of two methylcitrate cycle genes (
MoMCS1 and
MoMCL1) had been studied in
M. oryzae [
13]. The growth of Δ
Momcs1 on the media using propionyl-CoA-producing amino acids (isoleucine, valine, and methionine) as the sole amino acids was reduced, but to a lesser extent than Δ
Momcl1 [
13]. When culturing on the medium with glutamic acid or inorganic nitrogen NaNO
3 as a sole nitrogen source (not to produce propionyl-CoA directly), the growth of Δ
Momcs1 was normal, while the growth of Δ
Momcl1 was blocked [
13]. Δ
Momcs1 grew normally in minimal medium (MM) with glucose as the carbon and energy source, but grew slower in complete medium (CM) containing glucose and peptone. The growth of Δ
Momcl1 was slowed in both MM and CM media. The addition of 0.002% propionate to the MM medium further inhibited the growth of Δ
Momcl1 but not Δ
Momcs1. The growth of Δ
Momcs1Δ
Momcl1 in the MM medium and MM medium supplemented with 0.002% propionate was similar to Δ
Momcs1 but different from Δ
Momcl1. This difference in the growth phenotype of Δ
Momcs1 and Δ
Momcl1 in different carbon and nitrogen sources is related to the different intermediate metabolites accumulated in fungal cells: propionyl-CoA was accumulated in Δ
Momcs1 and Δ
Momcs1Δ
Momcl1 cells, while 2-methylisocitrate was accumulated in Δ
Momcl1 [
13].
3. Pathogenicity
In animal and plant pathogenic fungi, the methylcitrate cycle is required for pathogenic fungal virulence. However, knockout mutants of different genes in the pathway have different phenotypes, which are related to the type of intermediate compounds accumulated in the mutants. In
M. oryzae, knocking out
MoMCL1 resulted in a significant reduction in the virulence on plants, while the virulence of Δ
Momcs1 was normal [
13]. In
G. zeae, the virulence of Δ
Gzmcl1 to barley was weakened, while its virulence to wheat was normal [
16]. In
T. marneffei, a pathogen of fatal systemic fungal diseases, Δ
mcd (deletion of
MCD, a gene encoding a 2-methylcitrate dehydratase) showed an attenuated virulence in mice [
15].
A. fumigatus, which causes Aspergillosis in animals and humans, utilizes amino acids from the host as a source of nutrition. The 2-methylcitrate synthase (McsA) is essential for the invasive Aspergillosis, and Δ
AfmcsA have reduced virulence [
14,
19]. Moreover, the addition of sodium propionate to the culture medium killed the Δ
AfmcsA mutant [
19]. In a pathogenic fungus
Paracoccidioides lutzii, which causes Paracoccidioomycosis (PCM), a chemical compound (ZINC08964784) inhibits fungal growth by binding to the 2-methylcitrate synthase [
20].
Trichoderma atroviride, a kind of biological control fungi, can control the harm of
Botrytis cinerea and other pathogenic fungi. The inhibitory effect of Δ
Taicl2 (=Δ
Tamcl1) on the growth of
B. cinerea was decreased [
18].
4. Asexual and Sexual Reproduction
The methylcitrate cycle affects the asexual reproduction process of fungi. In
M. oryzae, the ability of Δ
Momcs1 and Δ
Momcl1 to produce spores was significantly reduced [
13]. In
A. nidulan, the addition of 20 mM propionate almost made Δ
AnmclA unable to produce spores [
17]. With regard to fungal sexual reproduction, the ability of Δ
Gzmcl1 to form perithecia is not affected in
G. zeae [
16].
5. Toxins and Melanin Synthesis
In
A. nidulans and
A. fumigatus, Δ
AnmcsA and Δ
AfmcsA produce fewer polyketide toxin (such as carcinogens, mycotoxins, and sterigmatocystin) and conidiospore pigment [
14,
21]. Propionyl-CoA-producing carbon or nitrogen sources such as propionate, heptadecanoic acid, isoleucine, and methionine inhibits polyketide and conidiospore pigment synthesis of
A. nidulans [
22]. The spore pigment synthesis of Δ
AnmcsA was blocked, and the color of the mutant's conidia changed from green or yellow to white [
10]. Adding exogenous propionate in the medium aggravated this mutant phenotype. In Δ
AnmcsA, excessive accumulation of acetyl-CoA inhibits the activity of polyketide synthase [
21]. Knockout of
PCSA—a gene encoding a putative propionyl-CoA synthase—in Δ
AnmcsA reduced the amount of intracellular propionyl-CoA and allowed the mutant to regain the ability to synthesize polyketides [
21].
6. Other Physiological Processes
In
M. oryzae, the ratio of NAD
+/NADH in the Δ
Momcs1 aerial mycelium decreased, and the content of nitric oxide (NO) also decreased, meaning that the methylcitrate cycle is involved in the cellular redox state and NO signaling [
13]. The altered NAD
+/NADH ratio may be related to the inhibition by propionyl-CoA of enzymatic activities of metabolic pathways such as the TCA cycle [
23]. In yeast
S. cerevisiae, propionic acid promotes endocytosis, and disrupts cell cycle and cellular respiration [
24].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28186667