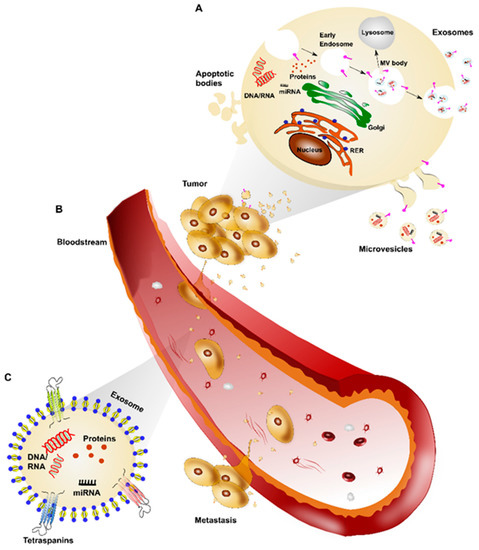
2. Exosomal Proteins in Breast Cancer Diagnosis
Proteins located on the surface of, as well as within exosomes, may also be used as cancer biomarkers. As shown by proteomic results available in the ExoCarta and EVPedia databases [
46], exosomes exhibit specific protein profiles according to cellular origin. As previously mentioned, tetraspanins are abundantly expressed in exosomes [
47]. These are a protein superfamily that interacts with a large variety of transmembrane and cytosolic signaling proteins [
48,
49]. In particular, tetraspanin CD9, along with metalloprotease ADAM10, heat-shock protein HSP70 and Annexin-1, are general marker proteins detected in serum and pleural effusion-derived exosomes from patients with BC or BC cell lines [
50]. Interestingly, Wang and colleagues [
51] recently showed that the level of exosomal tetraspanin CD82 was significantly higher in the serum of BC patients compared to healthy controls, while the expression of CD82 significantly increased with malignant breast cancer progression. Furthermore, the combined expression of urinary exosomal tetraspanin CD63 and miR-21 had a 95% sensitivity to early BC detection, although both markers are not specific to BC [
52].
Rupp et al. [
53] reported that the epithelial cell adhesion molecules EpCAM and CD24 could be used as markers to specifically identify cancer-derived exosomes in ascites and pleural effusions from BC and ovarian cancer. In the same period, Moon and colleagues [
54,
55] found that both plasma levels of developmental endothelial locus-1 protein (Del-1) and fibronectin expressed by circulating exosomes were significantly higher in patients with BC than in controls. Moreover, they almost returned to normal after tumor removal, proving to be closely related to tumor presence. Additionally, Khan et al. [
56] demonstrated that exosomal-Survivin, particularly Survivin-2B, may be employed as a diagnostic and/or prognostic marker in early BC patients.
Interestingly, exosomes from gastric, breast and pancreatic cancer carry members from the human epidermal growth factor receptor (HER) family [
57,
58,
59]. In HER2-overexpressing BC cell lines, HER2-positive exosomes modulate sensitivity to Trastuzumab and, consequently, HER2-driven tumor aggressiveness [
59]. Although not specific to early BC diagnosis, HER2 could be a useful biomarker for anticipating drug-resistance during treatment, which represents the principal limiting factor to the development of cures in cancer patients.
Additionally, Melo and colleagues [
60] identified a cell surface proteoglycan, glypican-1 (GPC1), specifically enriched on cancer cell-derived exosomes. They observed that GPC1-positive circulating exosomes were specifically and sensitively detectable in the serum of patients with pancreatic cancer. Elevated GPC1 levels have also been observed on exosomes from BC cells, suggesting a possible use of this exosomal biomarker to identify BC early [
61].
More recently, Kibria et al. [
62] used an automated micro flow cytometer to profile protein expression of exosomes isolated from cell lines and blood of BC patients and healthy controls. They observed a significant reduction in CD47 expression in circulating exosomes from BC patients, compared to controls. Notably, CD47 is a cancer-related surface protein whose expression prevents recognition of cancer cells by the innate immune system, thus facilitating tumor progression [
63].
Finally, other studies demonstrated the higher expression of serum exosomal-annexin A2 (exo-AnxA2) in BC patients compared to non-cancer females, especially for triple-negative BC (TNBC) rather than luminal and HER2-positive BC. Besides, high expression of exo-AnxA2 levels in BC was significantly associated with tumor grade, poor overall survival and poor disease-free survival. This study also showed that exo-AnxA2 promotes angiogenesis. Therefore, exo-AnxA2 represents a potential prognostic biomarker and therapeutic target of TNBC [
64].
3. Exosomal MicroRNAs in Breast Cancer Diagnosis
MicroRNAs (miRNAs) are short, noncoding single-stranded RNAs that regulate gene expression at a post transcriptional level by binding to the 3′ untranslated region of its target mRNA, leading to translational inhibition or mRNA degradation [
65]. Exosomes contain plenty of miRNAs [
66], and several studies investigated the role of exosomal miRNA expression in mediating biological effects in receiving cells [
67,
68,
69,
70,
71,
72]. In particular, miRNAs stably exist in body fluids by virtue of their packaging in exosomes, which protects them from degradation [
73]. Interestingly, exosomal miRNAs can act as novel ideal biomarkers in BC, because their expression profile correlates with tumorigenesis and tumor progression [
74,
75,
76,
77].
In 2016, Hannafon et al. [
78] showed that the levels of exosomal miR-21 and miR-1246 in plasma were markedly higher in BC patients than in healthy subjects. This suggests their potential use as biomarkers in BC, although miR-21 and miR-1246 are ubiquitous in human exosomes. These data are in keeping with other studies that described high levels of these miRNAs in serum or plasma from BC patients. In detail, Shimomura and colleagues [
79] evaluated serum miRNA expression profiles using highly sensitive microarray analysis, discovering a combination of five miRNA (miR-1246, miR-1307-3p, miR-4634, miR-6861-5p and miR-6875-5p) able to detect BC with high sensitivity, specificity and accuracy, even in the case of ductal carcinoma in situ (DCIS). Additionally, Fu et al. [
80] found that miR-382-3p and miR-1246 were significantly upregulated in the serum of BC patients, while miR-598-3p and miR-184 were significantly downregulated. Finally, a meta-analysis of Li and colleagues [
81] suggested that miR-21 is a potential biomarker for early diagnosis, with high sensitivity and specificity being significantly up-regulated in BC.
Although miR-145, miR-155, and miR-382 have been proposed as non-invasive biomarkers to distinguish BC patients from healthy individuals [
82], in 2019, Gonzalez-Villasana et al. [
83] isolated these miRNAs in the exosomes from serum of both BC patients and healthy donors. However, this study confirmed significantly higher concentrations of exosomes in BC patients compared to healthy donors, supporting the hypothesis of an association between exosome concentration and the presence of BC.
In another study of 50 BC cases and 12 healthy controls, Eichelser and colleagues [
84] reported that exosomal miR-101 and miR-372 were BC-specific, as confirmed by significantly higher serum levels in BC patients than in the control group. Moreover, Yoshikawa et al. [
85] showed that plasma exosome-encapsulated miR-223-3p levels may be a useful preoperative biomarker to identify invasive lesions in patients diagnosed with DCIS by biopsy. In particular, exosomal miR-223-3p level was significantly increased in BC patients compared to healthy controls and showed a significant correlation with histological type, pT stage, pN stage, pathological stage, lymphatic invasion and nuclear grade.
In 2019, in order to investigate the enrichment of exosomal miRNAs in the pathogenesis of BC and DCIS, Ni et al. discovered an increase of exosomal miR-16 levels in plasma of BC and DCIS patients compared to healthy women, especially in cases of luminal tumors. Moreover, lower levels of exosomal miR-30b were associated with recurrence, and exosomal miR-93 was upregulated in DCIS patients [
86].
In 2020, Zou et al. [
87] focused on tumor regulation roles of members from the miR-532-502 cluster. They analyzed the expression patterns of miRNAs in the miR-532-502 cluster in about 800 plasma and serum samples from BC patients and healthy controls. They found that three miRNAs (miR-188-3p, miR-500a-5p, and miR-501-5p) in plasma and five miRNAs (miR-188-3p, miR-501-3p, miR-502-3p, miR-532-3p, and miR-532-5p) in serum were significantly up-regulated in BC patients. Similarly, Li et al. [
88] identified five plasma miRNAs (let-7b-5p, miR-122-5p, miR-146b-5p, miR-210-3p and miR-215-5p) whose expression levels were significantly different in BC patients and controls. However, in plasma-derived exosomes, only miR-122-5p was consistently increased in BC patients. Moreover, evaluating the expression of 12 miRNAs from the miR-106a-363 cluster, the same authors [
89] also identified three plasma-derived exosomal miRNAs (miR-106a-3p, miR-106a-5p, and miR-92a-2-5p) and three serum-derived exosomal miRNAs (miR-106a-5p, miR-19b-3p, and miR-92a-3p), whose levels were upregulated in BC patients.
Recently, Zou et al. [
90] explored the expression of 12 miRNAs in 32 pairs of serum-derived exosome samples from BC patients and healthy controls, discovering 10 miRNAs (let-7b-5p, miR-106a-5p, miR-19a-3p, miR-19b-3p, miR-25-3p, miR-425-5p, miR-451a, miR-92a-3p, miR-93-5p, and miR-16-5p) to be consistently upregulated in serum-derived exosomes in BC patients compared to controls. Furthermore, Wang et al. [
91] found that total circulating miR-188-5p was abnormally elevated in BC patients, compared to both women with breast fibroadenoma and healthy subjects, and its level correlated with tumor stage. On the other hand, by analyzing exosomal miRNA only, miR-188-5p levels in the serum of BC patients were reduced compared to healthy controls. Moreover, they did not differ from patients with breast fibroadenoma.
Interestingly, Li and colleagues [
92] demonstrated that serum exosomal miR-148a levels were significantly downregulated in patients with BC as compared to healthy patients with benign breast tumors. Besides, the downregulation of serum exosomal miR-148a is closely associated with staging at diagnosis and disease relapse, indicating that it might be a promising non-invasive diagnostic and prognostic biomarker for BC. On the other hand, Rodriguez-Martinez and colleagues [
93] investigated the use of serum exosomal miRNAs as diagnostic biomarkers in 53 patients initially diagnosed with locally advanced BC. They discovered that before neoadjuvant therapy, exosomal miR-21 and miR-105 expression levels were higher in metastatic versus non-metastatic patients and healthy controls. Based on these results, the authors suggested adding miR-21 and miR-105 analysis to mammogram tests, in order to identify those patients with metastatic disease who are misdiagnosed as non-metastatic by current clinical methods.
Some of the above studies demonstrated a different miRNAs’ expression profile according to tumor subtype. More specifically, exosomal miR-373 serum level was more elevated in case of TNBCs than in luminal cancers or unaffected patients [
84]. Likewise, higher levels of serum exosomal miR-222 were observed in basal-like and in luminal B versus luminal A tumor subtypes [
93]. In addition, the analysis of exosomal miRNA from BC cell lines using a Surface-Enhanced Raman Scattering (SERS) sensor confirmed a significantly higher expression of miR-21 in luminal and TNBCs compared to HER2-positive BCs, as previously reported [
94]. On the other hand, miR-222 was detected in TNBCs and high level of miR-200c was observed in HER2-positive BCs [
95]. Moreover, for the purpose of identifying particular miRNA signatures in exosomes derived from plasma of HER2-positive BC and TNBC patients, Stevic and colleagues [
96] discovered 10 miRNAs (miR-27a/b, miR-30c, miR-150, miR-152, miR-199a-3p, miR-340, miR-376a, miR-410, and miR-598) in the entire cohort of BC patients, 13 miRNAs (miR-27a/b, miR-30c, miR-150, miR-152, miR-199a-3p, miR-328, miR-340, miR-365, miR-410, miR-422a, miR-598, and miR-628) in a subgroup of 211 HER2-positive BCs, and 17 miRNAs (miR-27b, miR-30c, miR-128a, miR-145, miR-150, miR-152, miR-199a-3p, miR-324-3p, miR-335, miR-340, miR-376a/c, miR-382, miR-410, miR-423-5p, miR-433, and miR-598) in the subgroup of 224 TNBC, significantly deregulated.
Based on a case-control study of 69 BC patients vs. 40 healthy controls, interestingly, Hirschfeld and colleagues [
97] have recently identified a specific panel of four urinary microRNA (miR-424, miR-423, miR-660, and let7-i) as a highly specific combinatory biomarker tool discriminating BC patients from healthy controls, with 98.6% sensitivity and 100% specificity.
Studies of exosomal miRNA detected in serum and plasma of BC patients and potentially useful for early diagnosis are summarized in Table 1. To date, numerous studies on exosomal miRNAs linked to tumors, and BC in particular, have been published. This number is destined to increase, given the growing curiosity and attention toward this new potential diagnostic and prognostic tool. However, further research is needed in order to identify the most focused and promising set of miRNAs.
Table 1. Exosomal miRNA detected in serum or plasma of BC patients that could be useful for early diagnosis.
| miRNA |
Special Characteristics |
References |
| miR-21 and mi-R1246 |
Significantly high in BC although ubiquitous in human exosomes |
Hannafon et al. [78] |
| miR-145, miR-155 and miR-382 |
Significantly high in BC although ubiquitous in human exosomes |
Gonzalez-Villasana et al. [83] |
| miR-101 and miR-372 |
Significantly high in BC |
Eichelser et al. [84] |
| miR-223-3p |
Significantly high in BC |
Yoshikawa et al. [85] |
| miR-16 |
Significantly high in BC, especially if estrogen-positive |
Ni et al. [86] |
| miR-93 |
Significantly high in DCIS |
Ni et al. [86] |
| miR-188-3p, miR-500a-5p and miR-502-3p (miR-532-502 cluster) |
Significantly high in BC |
Zou et al. [87] |
| miR-122-5p |
Significantly high in BC |
Li et al. [88] |
| miR-106a-3p, miR-106a-5p, miR-92a-2-5p, miR-19b-3p and miR-92a-3p (miR-106a-363 cluster) |
Significantly high in BC |
Li et al. [89] |
| let-7b-5p, miR-106a-5p, miR-19a-3p, miR-19b-3p, miR-25-3p, miR-425-5p, miR-451a, miR-92a-3p, miR-93-5p and miR-16-5p |
Significantly high in BC |
Zou et al. [90] |
| miR-148a |
Significantly downregulated in BC |
Li et al. [92] |
| miR-27a/b, miR-30c, miR-150, miR-152, miR-199a-3p, miR-340, miR-376a, miR-410 and miR-598 |
Significantly deregulated in BC |
Stevic et al. [96] |
| miR-21 and miR-105 |
Significantly high in metastatic vs. localized BC |
Rodriguez-Martinez et al. [93] |
| miR-373 |
Significantly high in TNBC |
Eichelser et al. [84] |
| miR-222 |
Significantly high in TNBC and luminal B vs. luminal A BC |
Rodriguez-Martinez et al. [93] |
| miR-27b, miR-30c, miR-128a, miR-145, miR-150, miR-152, miR-199a-3p, miR-324-3p, miR-335, miR-340, miR-376a/c, miR-382, miR-410, miR-423-5p, miR-433 and miR-598 |
Significantly deregulated in TNBC |
Stevic et al. [96] |
| miR-27a/b, miR-30c, miR-150, miR-152, miR-199a-3p, miR-328, miR-340, miR-365, miR-410, miR-422a, miR-598 and miR-628 |
Significantly deregulated in HER2-positive BC |
Stevic et al. [96] |