Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
Pathogens and pests constantly challenge food security and safety worldwide. The use of plant protection products to manage them raises concerns related to human health, the environment, and economic costs. Basic substances are active, non-toxic compounds that are not predominantly used as plant protection products but hold potential in crop protection. Basic substances’ attention is rising due to their safety and cost-effectiveness.
- disease management
- pest management
- sustainable crop protection
- integrated pest management
- organic farming
1. Introduction
The use of plant protection products, such as fungicides, insecticides, and herbicides, is crucial for controlling diseases and pests in agriculture, but their safety, costs, and availability are a growing concern [1,2,3,4]. The possible adverse effects on human and environmental health have led to the development of risk exposure indicators [5,6] and more stringent legislative requirements [7,8]. The EU, for example, regulates plant protection products authorization [9] and utilization to endorse a new paradigm for agricultural production with the transition to low-input farming, promoting integrated pest management and complementary alternatives to minimize the utilization of plant protection products [10]. The use of plant protection products has negative impacts, in terms of their direct costs and negative externalities, on producers and the environment, especially in developing countries [11]. While ensuring rigorous testing for safety and quality, the product registration process increases the costs of developing new products and lengthens the time to market [12,13]. Additionally, the shift towards single-site compounds, which have a more favorable profile than multi-site compounds, increases the risk of resistance development in pests and pathogens [14].
Basic substances can represent an opportunity to mitigate the problems associated with traditional plant protection products. Basic substances are defined as compounds that are not predominantly used as plant protection products but may be useful in crop protection. They have no toxicological concerns and do not cause adverse effects on humans, animals, or the environment [9]. Interestingly, ‘foodstuff’ substances (as defined by Regulation (EC) No. 178/2002) are intrinsically considered basic substances [15]. Basic substances have no residue limits, and usually no pre-harvest interval [16]. Also, since they are not currently placed on the market as plant protection products, they are not considered in the Harmonized Risk Indicator 1 calculation that is used in the EU for highlighting the trends in the risks associated with the use of pesticides [17]. European basic substances partially overlap with the American “Generally Recognized as Safe” (GRAS) substances, which are approved for use in food products as preservatives [18].
2. Activity of Approved Basic Substances against Fungal Diseases
Fungicides have been the top-selling group of plant protection products in the EU for a long time. Three countries, namely Spain, France, and Italy, make up around 62% of the total volume of pesticides (330 thousand tons) sold annually between the years 2011 and 2020 [28]. Interestingly, these countries also have the highest surface area dedicated to viticulture, which accounts for 75% of the 3.2 million hectares under vines [29]. This is due to the fact that many fungicide sprays are applied each growing season to the grapevine crop (Vitis vinifera) to manage three major fungal diseases: downy and powdery mildews, and grey mold [30]. The major fungicide markets are fruit and vegetables, cereals, grapevines, and potatoes, which account for about 60% of the global fungicide market (https://www.apsnet.org/edcenter/apsnetfeatures/Pages/Fungicides.aspx, accessed on 3 May 2023). In the following paragraphs, information on the results achieved through basic substance applications to control the important diseases of grapevines, potatoes, and fruits, in general, will be provided, taking into consideration that pre-harvest treatments also affect the post-harvest control of the pathogens (Table 1).
2.1. Grapevine
2.1.1. Grapevine Downy Mildew
European grapevine exhibits a high level of susceptibility to grapevine downy mildew, caused by the oomycete Plasmopara viticola [31]. To prevent infections and the consequent production loss, several treatments with chemical fungicides are needed during the season under both organic and integrated pest management systems [31]. This results in negative consequences for the environment and risks for human health. Copper is the most widely applied plant protection product acting against grapevine downy mildew, although the Regulation (EU) 2018/1981 restricted the quantities allowed and classified this heavy metal as an active substance candidate for substitution [32]. Copper fungicides are fundamental for organic productions, where the use of synthetic curative compounds is not allowed, but also play a central role in integrated pest management to limit the outbreak of resistant strains. This situation encouraged the search for alternative tools to protect plants from P. viticola. Chitosan [33], biocontrol agents [34], aptamers [35], hydrolyzed proteins [36], laminarin [37], stilbenes [38], and other plant extracts [39,40,41] showed promising results under in vitro or in vivo experiments. Among these alternatives, basic substances could present a good opportunity. Chitosan, Equisetum arvense (horsetail), sucrose, Salix spp. cortex, lecithin, fructose, and nettle (Urtica spp.) are the basic substances that may exhibit effectiveness against grapevine downy mildew [16], especially when integrated into reduced copper strategies. The field application of chitosan hydrochloride alone showed promising results in plot trials, under different environmental conditions, and even under the presence of a high disease pressure [22,42,43]. This biopolymer is obtained from chitin deacetylation, and it can perform eliciting, antimicrobial, and film-forming activities once applied on plant tissues [22]. Results obtained with chitosan individual treatments were similar to those obtained with a conventional application of copper, showing disease reductions compared to the untreated control, which in some cases exceeded 95% on leaves and 80% on grape bunches [22,42]. Nevertheless, chitosan effectiveness against grapevine downy mildew is strictly linked to two main factors: volume of applications and active ingredient concentrations. To best perform the triple mode of action on plant tissues, a good wetting of the canopy is required and the standard spraying volume for grapevine (1000 L/ha of water) is recommended. Application of 0.8% chitosan has been found to perform better than copper hydroxide in seasons characterized by frequent rainfall and high disease pressure [42]. The 0.5% of active ingredient does not usually show significant differences compared to the 0.8% in terms of their efficacy, as well as being less expensive for the growers. Furthermore, treatments with high concentrations of chitosan for the whole season can induce undesired collateral physiological responses in vines, such as reduced growth and leaf area [42]. In addition to being dangerous for humans and ecosystems [44], copper residues on the berries affect the wine quality, reducing the concentration of several amino acids in the must [45]. Unlike copper, chitosan and other natural compounds, such as laminarin, have lower impacts on the final product quality [45]. Results obtained in the past years have suggested chitosan as a promising tool to support or eventually replace copper for grapevine downy mildew management. Copper and chitosan could even coexist to begin with, for example with alternating or combined treatments, even if validations on a commercial scale for these strategies are needed. According to the data available in the literature, no copper could be applied under instances of low disease pressure, while a valid strategy for difficult seasons could be to apply copper until flowering (in the period of higher susceptibility) and then replace it with chitosan. In this way, it could be possible to reduce the quantities of copper distributed per year on the one hand and the costs of chitosan on the other hand. Indeed, the main limitations regarding chitosan diffusion so far are represented by its cost and the lack of operational knowledge. It will be important to invest in new formulations and to investigate the miscibility of this biopolymer with other plant protection products, since farmers are used to simultaneously applying several compounds so as to target different pests within a single treatment.
2.1.2. Grey Mold on Table Grape
Grey mold is a globally widespread and economically relevant disease of grapes caused by the second most important phytopathogenic fungus, Botrytis cinerea [46]. This broad host range pathogen affects several crops, both under pre- and post-harvest. B. cinerea can survive and develop in vineyards as both a necrotrophic pathogen and a saprophyte [47,48]. Grey mold can result from multiple infection pathways on ripening grape berries, including latent infections established during blooming, direct berry infection due to airborne conidia, and berry-to-berry infection caused by mycelium originating from previously infected berries (nesting path) within the cluster [49,50], which spread according to a nesting path. Although B. cinerea causes about 30% of latent infections [51], it is difficult to precisely estimate the global losses due to its broad host range and specific missing statistics. New Zealand recorded costs due to grey mold direct crop losses and grey mold control measures of up to NZD 5000/ha and NZD 1500/ha, respectively, in growing seasons favorable for disease development [47]. In Australia, Chile, and South Africa, grey mold is the main cause of wine and table grape losses, from the vineyard to the retail outlet, entailing profit reductions of AUD 52 million/year, USD 22.4 million/year, and ZAR 25 million/year, respectively [46]. Chemical fungicides are the most important control means available, although fungicide resistance is an increasing issue for this pathogen [52,53,54]. Fungicide-resistant phenotypes were detected in B. cinerea populations in table grape vineyards in California, with genotypic resistance against boscalid, cyprodinil, fenhexamid, and pyraclostrobin in 95%, 85%, 23%, and 14% of tested isolates, respectively [55]. Differences in the fungicide resistance profile of B. cinerea may be due to the species/groups included within the complex; as an example of biodiversity, in the pomegranate fruit, B. cinerea, B. pseudocinerea, and Botrytis group S were the etiological agents of grey mold [56,57]. Currently, latent infections caused by Botrytis spp. are completely prevented in storage through the use of SO2-generating pads [51], although these entail adverse effects on food, humans, and the environment (i.e., phytotoxicity, development of antimicrobial resistance, allergy, pollution, etc.) [58], and cannot be applied to organic table grapes. This encourages the set-up of new, safer, more effective, and cheaper alternative control means and strategies. Basic substances, such as salts and chitosan, and potential basic substances can be a promising alternative to chemical fungicides for grey mold management [59,60]. Chitosan treatments have been shown to significantly reduce disease incidence both in the field and after harvest. It indirectly enhances the activity of the key plant enzymes involved in disease resistance, such as superoxide dismutase, peroxidase, catalase, and ascorbate peroxidase, that damage the mycelial structures of Botrytis spp. and reduce pathogen development [61,62,63].
Grey mold on table grapes is a disease affecting clusters both in the field and during the post-harvest phases. Unfortunately, since B. cinerea affects grapes more heavily during the post-harvest phases, most of the papers that are available on this subject concern the disease development after harvest, and very few concern pre-harvest evaluations [64]. Grey mold protection starts during the grapevine growing season following a well-established scheme, in which four applications of fungicides are carried out under the following specific phenological stages: berry set, pre-bunch closure, veraison, and 1–3 weeks before harvest. This strategy, that is mandatory to avoid latent infections during the growing season [50], was also adopted for chitosan and other alternative control means. In field treatments on table grapes, 1% chitosan demonstrated the same ability to protect grapes from grey mold as the strategy based on synthetic fungicide application. In an integrated program lasting two years, chitosan-treated “Chardonnay” wine grapes exhibited a degree of disease severity at harvest that was more than halved compared to the untreated control and was as effective as the synthetic fungicide program [65]. Chitosan has also been combined with active antimicrobial substances, such as essential oils, and applied as pre-harvest treatments [66] or as post-harvest coatings to improve the preservation of qualitative parameters and reduce the product losses caused by Botrytis spp. A possible evolution in the application of chitosan is its formulation as nanoparticles, which in preliminary trials behaved better than standard formulations [63]. This basic substance has been used formulated as a chitosan/silica nanocomposite-based compound, which reduced conidial germination and germ tube elongation, affecting the development of grey mold on the grapes [63]. Various substances have been tested at pre-harvest in combination with chitosan, such as with chitosan added or complexed with salicylic acid. In particular, the CTS-g-SA complex improved fruit physiology (transpiration and respiration rates), qualitative parameters (soluble solids, titratable acidity, and total phenolic content), and defense mechanisms involving the control of disease incidence [58].
Among the other basic substances currently approved, sodium bicarbonate has been studied worldwide, leading to results that are, in many cases, not different, if not better, than the synthetic fungicides [67]. When applied before harvest, sodium bicarbonate showed a significant reduction in botrytis storage rots in both small-scale and large-scale tests. In large-scale trials simulating practical commercial conditions adopted in Southern Italy, two salt applications (at 30 and 90 days before harvest) of sodium bicarbonate significantly reduced grey mold from 23% (untreated control) to 12% [68]. Among these basic substances, sodium bicarbonate may represent one of the most useful and effective compounds, considering that it is easy to find on the market, is very cheap, has a broad spectrum of activity against a variety of pathogens, is well accepted by consumers and operators, and has an acceptable environmental profile.
2.2. Potato Leaf Diseases
The potato (Solanum tuberosum) is one of the most important vegetable crops in the world. It belongs to the family Solanaceae and is an important starchy food crop. Potato plants are subjected to numerous diseases wherever the crop is grown. Among the approved basic substances, there are some that have the potential to limit the early and late blight of potatoes by spraying or dipping tubers before sowing. Early blight of potatoes caused by Alternaria solani and Alternaria alternata, and late blight caused by the oomycete Phytophthora infestans are major causes of concern in potato production. This problem is particularly important in organic farming, where synthetic fungicides are prohibited. Therefore, a necessary condition in the organic cultivation of potatoes is the timely implementation of treatments and adherence to the rules of agricultural technology regarding the appropriate variety and crop rotation.
Alternaria spp. are air- and soil-borne organisms that cause disease on foliage (leaf blight), stems (collar rot), and tubers (tuber rot), resulting in severe damage during all stages of plant development [69]. This disease causes losses in crop productivity in the field and in tuber quality during storage. The average annual yield loss of potatoes due to this disease is approximately 79% of the total production, depending on the nature of the disease, weather conditions, and the type of variety grown [70]. It can destroy foliage prematurely and in a short time, reducing production, while the tuber infections associated with rots can cause significant crop losses during storage. It is considered one of the most destructive crop pathogens threatening global food security. In organic potato production, late blight can cause severe losses in the potato yield and quality. Currently, in organic farming it can only be effectively controlled using copper fungicides. However, some countries prohibit copper use in organic farming based on their national laws, as the harmfulness of copper in ecosystems is still being debated. Studies aiming at the reduction in copper usage and testing of potential basic substances against late blight for organic farming are needed. Currently, basic substances, such as extracts from the bulb of the onion crop (Allium cepa) and horsetail, have been proposed as protective treatments against early blight. In the case of P. infestans, chitosan hydrochloride is most frequently mentioned as an elicitor, along with nettle extracts, lecithins, and dried horsetail [16]. However, the use of these substances in the field has not often been demonstrated. The application of elicitors and botanical fungicides, beneficial microorganisms, and basic substances should be a combination of compounds and microorganisms with different modes of action, beginning at the early stages of the potato plant’s growth [71,72,73,74]. This strategy of minimizing the risk to the ecosystem is a global trend, especially in the EU, where the policy of greening agriculture is being promoted.
Chitosan significantly inhibits the mycelial growth and in vitro spore germination of P. infestans, induces resistance to the pathogen in potato pieces and leaves [75], and forms a mechanical barrier to the pathogen penetration [76,77]. It also has a synergistic effect with plant protection products, making it a potential way to reduce the use of chemical plant protection products. In field conditions, the use of chitosan can stimulate plants to defend themselves, which in turn contributes to limiting the harmful effects of potato disease symptoms. Late blight epidemics were delayed on plots that received eight sprays of 0.1% chitosan [78] and provided 60% protection against late blight by mixing 4% chitosan with a plant elicitor [79]. Some late blight reducing potential for 0.4% chitosan was found in field tests performed in Germany on the cultivars Nicola and Ditta [80]. Field tests confirmed some of the major results coming from the lab and growth chamber assays. Most effects were only visible during the early phases of the disease, when plants were still vigorous, but might have been more pronounced under a different infection regime with an earlier onset of the disease. Both in full-field tests, chitosan (0.4%) and the copper fungicide, and in a small plot trial, chitosan (0.4%) accompanied with the horsetail and liquorice products seemed to be able to cause some degree of disease reduction, even under an extremely late infection regime [80]. Good results with a low-level copper formulation (copper sulfate pentahydrate), together with chitosan as an adhesive substance to increase rain fastness, were also obtained [81]. In recent years, practical applications of chitosan were tested against P. infestans in in vivo experiments under outdoor conditions [82]. This experiment showed that chitosan is very effective against P. infestans. An average damage of over 76% was observed in the control plants. In the treated variants with 1–4 applications of chitosan, the final damage to the plants ranged from 48% to 0.5%. Expressed as values of the final inhibitory protective effect, a single application of a 0.4% solution of chitosan provided an inhibitory effect of 37%. In cases where chitosan was applied four times, an inhibitory effect of up to 99.3% was demonstrated [82]. The newest study also confirmed that chitosan can be applied as the nano compound. The bioactivity and absorbency of elicitors are critical factors that limit the large-scale field application. A star polymer was constructed to deliver the nano-sized (particle size from 144.61 nm to 17.40 nm in an aqueous solution) chitosan to enhance the control effects against potato late blight [83].
As basic substances, lecithins have fungicidal activity due to their inhibition of the fungal hypha penetration into the plant cells. Unlike chitosan, lecithins are not fully soluble in water. Since chitosan would be the first choice in most situations when looking for a fungicide among the basic substances, the next option for controlling oomycetes may be to use lecithins in combination with chitosan in joint field treatments (https://eutrema.co.uk/basic-substances-what-are-they-and-how-can-they-used-for-pest-and-disease-control-on-farms/, accessed on 20 January 2023). The fatty acids present in the lecithins could act more positively via plant defense stimulation, rather than through a toxic effect. In fact, linolenic acid and its precursor linoleic acid, both present in soy lecithin, are the precursors of a wide variety of oxylipins and the plant hormone jasmonic acid, which actively participate in plant defenses [84]. Lecithins have not been studied in field trials.
In field tests, the application of 12 kg/hL horsetail macerate showed effectiveness in protecting the tomato crop (Solanum lycopersicum) from late blight that was analogous to the copper-based treatments [20].
Nettle slurry (Urtica dioica), used as a foliar fertilizer in different doses, alone or in combination with horsetail, had no significant effects on the yield, chlorophyll content, or the presence of pests and diseases in organic potato crops [85]. Conversely, the methanolic leaf extracts of nettle slurry and broad-leaf hopbush (Dodonaea viscosa) demonstrated a strong antifungal efficacy against A. alternata. Among the many polyphenolic compounds that were detected in the HPLC of the extract, coumaric acid, caffeic acid, ferulic acid, and α-tocopherol showed potent in vitro fungicidal activity against A. alternata, either applied alone or in combination at low concentrations [86].
In Romania, 2.2% and 3.3% water solutions of the onion crop showed significant protection against A. solani in potato fields [87]. Dry extracts of onion (concentration 20.0 mg/mL) showed antifungal activity against A. alternata and P. infestans. In particular, red onion extracts showed a higher efficacy in inhibiting A. alternata than white onion extracts, which showed no efficacy. This result is surprising, considering that both extracts have a similar amount of quercetin, an antioxidant with antifungal activity. Evidently, other components of these extracts are responsible for the A. alternata inhibition [88].
2.3. Pre-Harvest Treatment Affecting the Post-Harvest Diseases of Fruits
Fruit-bearing plants may be infected in the field before or during their harvest, providing inoculum for post-harvest decay following their harvest [89]. The accumulation and/or survival of the inoculum can be prevented through pre-harvest treatments [90]. Basic substances may provide environmentally friendly alternatives to pre-harvest fungicide application to prevent the post-harvest decay of fruits and vegetables, although there is limited information about their efficacy.
Chitosan hydrochloride and chitosan are the most widely studied basic substances in pre-harvest application, either alone or in combination. Similarly to what has been observed for table grapes [91,92,93,94], the pre-harvest application of 0.2–1% chitosan was effective against grey mold latent infection and the decay of strawberries (Fragaria x ananassa and Fragaria chiloensis) [95,96,97,98,99]. The pre-harvest application of 1% chitosan was effective against the grey mold and brown rot of sweet cherries (Prunus avium) and date palm fruits (Phoenix dactylifera) [100,101]. The soft rot of kiwifruit (Actinidia deliciosa) caused by Botryosphaeria dothidea and Phomopsis sp. was also reduced following a chitosan-containing spray [102]. This basic substance was effective in being able to reduce the A. alternata-related decay of apricots (Prunus armeniaca) [103,104] and in the decay of peaches (Prunus persica) [105,106]. The pre-harvest treatment of jujube (Zizyphus jujuba) and tomato plantations with 0.3–1 g/L chitosan was also effective in being able to reduce the decay of these harvested fruits [107,108]. However, in the case of raspberries (Rubus idaeus), 1% or 2% chitosan was only effective in reducing the decay of these fruits during their storage [109].
Table 1. List of the basic substances which effectively protected the crops described herein from specific diseases.
| Crop (Species) | Disease (Pathogen) | Basic Substance | Reference |
|---|---|---|---|
| Grapevine (Vitis vinifera) | Downy mildew (Plasmopara viticola) | Chitosan | [42,43] |
| Botrytis bunch rot (Botrytis cinerea) | Chitosan | [66] | |
| Potato (Solanum tuberosum) | Early blight (Alternaria alternata) | Nettle slurry (Urtica dioica) and broad-leaf hopbush (Dodonaea viscosa) methanolic extracts | [86] |
| Early blight (Alternaria solani) | Water solutions of Allium cepa | [87] | |
| Late blight (Phytophthora infestans) | Chitosan | [78,79,80,82] | |
| Strawberry (Fragaria x ananassa and Fragaria chiloensis) | Grey mold (Botrytis cinerea) | Chitosan | [90,95,96,97] |
| Sweet cherry (Prunus avium) | Storage decay | Chitosan | [98] |
| Botrytis rot (B. cinerea) | Sodium bicarbonate salts | [108] | |
| Date palm fruit (Phoenix dactylifera) | Storage decay | Chitosan | [99] |
| Kiwifruit (Actinidia deliciosa) | Soft rot (Botryosphaeria dothidea and Phomopsis sp.) | Chitosan | [100] |
| Apricot (Prunus armeniaca) | Decay (A. alternata) | Chitosan | [101,102] |
| Peach (Prunus persica) | Decay (A. alternata) | Chitosan | [103,104] |
| Jujube (Zizyphus jujuba) | Storage decay | Chitosan | [105] |
| Tomato (Solanum lycopersicum) | Storage decay | Chitosan | [106] |
| Pear (Pyrus communis) | Storage decay | Onion (Allium cepa) extract | [109] |
The pre-harvest application of other basic substances has received fewer widespread studies. The botrytis rot on sweet cherries was reduced by spraying sodium bicarbonate salts, even if with a far lower effectivity than under post-harvest application [110]. Three applications of onion extract on pear trees (Pyrus communis) decreased the decay of the stored fruits [111].
The pre-harvest effectivity of basic substances with effective control of post-harvest pathogens, such as horsetail extract that protects from post-harvest pathogens, like B. cinerea, C. acutatum, and Monilinia sp. [16], should also be studied in the future.
The pre-harvest usage of basic substances provides a promising alternative to fungicides acting against different post-harvest pathogens. However, their application must be optimized for different plant products and conditions. Their effect, for instance, can be further increased in combination with different substances, like calcium, salicylic acid, or methyl jasmonate [100,101,103,104,105,112,113,114].
3. Activity of Approved Basic Substances against Insects
Based on the analysis of data obtained from Scopus, over 3000 articles regarding eco-friendly natural product pesticides in crop protection have been published in the Agricultural and Biological Sciences sector, with an increasing trend in the last 30 years and a peak of 271 papers published in 2021. However, a search on Scopus using the keywords ‘pest control’ and ‘basic substance’ yielded only 11 scientific articles published since 2015, with the first article published in 2015 [15]. According to the EU Regulation (EC) 1107/2009, among the twenty-four basic substances permitted for plant protection use, eight are approved as insecticides (nettle, sodium chloride, L-cysteine, sucrose, and fructose), physical barriers (talc E553B), attractants (diammonium phosphate), or repellents (onion oil). In the following paragraphs, the literature concerning the field application of these eight basic substances will be described, along with their modes of action (Figure 2).
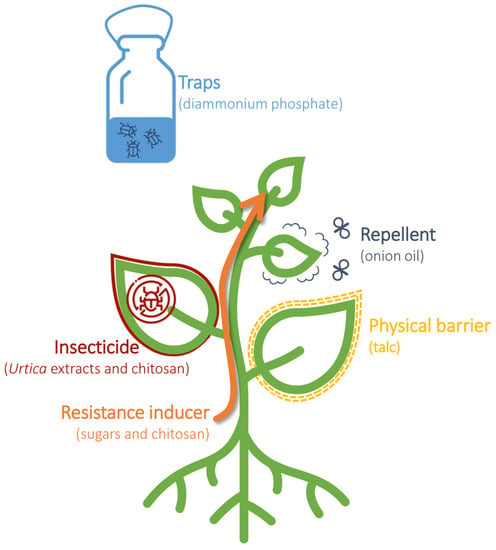
Figure 2. Utilization of basic substances in the management of plant pests. Basic substances can be used as insecticides, resistance inducers, physical barriers, repellents, and traps to control and manage plant pests.
3.1. Nettle
The extracts of nettle, commonly known as a foodstuff and medicine, are traditionally used by farmers who claim a significant reduction in the aphid and Coleoptera presence [115,116,117]. Searching the keywords “Urtica” and “pest management” on the Scopus database resulted in the retrieval of more than 500 papers that were published in the Agricultural and Biological Sciences sector, demonstrating that nettle is one of the most studied basic substances for pest management purposes. Nettle can be used as a fermented aqueous extract in spray applications against different aphid species, such as Myzus persicae, Macrosiphum rosae, Eriosoma lanigerum, and Panaphis juglandi, to protect fruit trees (Malus domestica, Prunus spp.), elder trees, beans (e.g., Phaseolus vulgaris), leafy vegetables (Lactuca sativa, Brassica oleracea), Rosa spp., and Spiraea spp. With a population density reduction of more than 30%, nettle extracts can also be used on Brassicaceae crops against the flea beetle, Phyllotreta nemorum, and the diamond back moth, Plutella xylostella, as well as on apple and pear trees against the codling moth, Cydia pomonella. In field trials, nettle slurry fermented extract showed a repellent activity towards Hyalopterus pruni and P. juglandi [118,119], but not against Aphis spiraephaga [118], suggesting that the efficacy of the nettle slurry extract against aphids is species-dependent. Under controlled conditions, Urtica urens water extract effectively limited the fertility of M. persicae, slightly reducing the increase of its population (by 20% on average), while no negative effects were registered on its natural enemy, Macrolophus pygmaeus [115]. Furthermore, the nettle extract used in combination with other biorational insecticides could improve the efficacy against aphid pests [115]. Nettle extracts can also be used to control the mites Tetranychus urticae on beans and Tetranychus telarius on grapevines. Repellent, acaricidal, and antifeedant activities of the nettle extracts against T. urticae, one of the economically most important pests in a wide range of outdoor and protected crops worldwide, have been reported [120,121]. Less information is available in the literature regarding the effect of nettle extracts on T. telarius. In this scenario, extracts from several plants proved to exert insecticidal or miticidal activity against vegetables and stored-product pests [122,123,124,125], that, in some cases, were comparable to those achieved using chemical insecticides (e.g., synthetic pyrethroid) [126], and could suggest potential basic substances as alternatives to synthetic chemical insecticides in crop protection.
3.2. Sucrose and Fructose
Sucrose and fructose are involved in the phenomenon of “Sweet Immunity”, according to which the sugar metabolism and signaling influence the plant immunity networks [21,127,128]. The quantities and ratios of three soluble carbohydrates (sucrose, D-fructose, and glucose) and three sugar alcohols (sorbitol, quebrachitol, and myo-inositol) of apple tree surfaces play a role in the trees’ resistance, as they influence the host preference, egg laying, and the behavior of the neonate larvae of Cydia pomonella [129,130,131,132]. Recent studies demonstrated that sucrose, in micro-dose foliar applications, can induce partial resistance via antixenosis to C. pomonella egg laying [133]. Moreover, the spraying of glucose or fructose significantly reduced the percentage of damaged fruits by C. pomonella by 70% compared to the untreated control, with an effectiveness comparable with the spraying of the chemical insecticide deltamethrin [134,135].
In field trials, sucrose treatment was found to be as efficient as thiacloprid treatment in the reduction of damage by C. pomonella. Furthermore, synergistic effects were found when sucrose was combined with the thiacloprid insecticide [133], and between fructose and organophosphorus or insect growth regulator insecticides against the codling moth [136].
The quantities and ratios of soluble carbohydrates and on the leaf surface could also influence the egg-laying preferences of Ostrinia nubilalis on maize hybrids [137,138,139,140,141]. A study contributed to explore the efficacy of sucrose and fructose, used alone or in combination with natural pyrethrum, against O. nubilalis and Scaphoideus titanus [142]. The authors found that the application of sucrose associated with fructose provided the best efficacy in reducing the number of corn borer larvae per plant with a 23% efficacy. In the case of S. titanus, sucrose seemed to increase the action of natural pyrethrum, whilst the fructose showed the same efficacy as the natural pyrethrum.
Finally, sugars could be also interesting as components of commercial biopesticides due to the phagostimulant activity for a more effective ingestion by larvae [143]. These studies demonstrate a promising alternative to conventional crop protection tools [144] and pave the way for the development of eco-friendly control strategies using the new concept of “Sweet Immunity” induction.
3.3. Talc
Magnesium hydrogen metasilicate, known by the common name of talc, is approved as a basic substance to be used in outdoor applications on grapevines and fruit orchards to act as a physical barrier towards insects and mites, like Cacopsylla pyri, Cacopsylla fulguralis, Drosophila suzukii, Panonychus ulmi, and Bactrocera oleae [145,146,147].
Nowadays, the research interest in the use of inert dusts and their potential role in agriculture to manage diseases and protect crops from insect pests is increasing [148,149]. Among mineral products, natural zeolites, a broad range of crystalline hydrated aluminosilicates [150,151], could represent potential basic substances. Thanks to their physical and chemical properties and uses, the Codex Alimentarius Commission (1999) endorsed their use for pest control in food commodities and listed zeolites as granted substances in the organic food production and plant protection [152]. The insecticidal activity of zeolites towards stored-product insect pests, such as Sitophilus zeamais, Rhyzopertha dominica, Sitophilus oryzae, Tribolium castaneum, Lasioderma serricorne, Tribolium confusum, Callosobruchus maculatus, and Meligethes spp., was intensively reported [149,153,154,155,156,157,158,159,160,161,162]. In addition, the 40% reduction in the oviposition rates of B. oleae females due to zeolite applications was observed [163].
3.4. Diammonium Phosphate
Plant volatile compounds are involved in the host-finding process and oviposition site selection by insects [164,165]. The efficiency of traps used in indirect (e.g., monitoring) and direct (e.g., mass trapping, attract, and kill) semiochemical-based control tools can be improved significantly through the addition of certain food attractants [166]. Ammonia-releasing substances play an important role in both sexes of fruit fly attraction to food sources [167,168,169]. Thus, ammonia bait traps are currently used for monitoring fruit fly populations [170]. The use of diammonium phosphate is permitted to bait one trap per tree in orchards, including Prunus spp., Citrus spp., and olives (Olea europaea), to enable the massive capture of adults of the above-mentioned insect species. In this context, fruit fly pheromones added to food attractants, such as diammonium phosphate, are efficient for the monitoring and mass trapping of C. capitata, B. cucurbitae, and B. dorsalis [171], and are commonly used in the monitoring of B. oleae in most olive-growing countries of the Mediterranean basin [172].
3.5. Onion Oil
Onion oil obtained from A. cepa is authorized as a basic substance due to its repellent and scent masking activity against the carrot root fly, Psila rosae [173]. Dispensers of undiluted oil placed in the field are able to disorient adult flies which cannot find its host plant. Dispensers are filled with onion oil alone or with ethylene vinyl acetate granules that are able to improve the release of vapor.
3.6. Chitosan
Among these basic substances, chitosan stimulates the defense system of crops against several classes of pathogens, including fungi, viruses, bacteria, and phytoplasmas [22], and its use as an elicitor of the crop’s self-defense mechanisms has also been approved. Chitosan also exhibits a strong level of insecticidal activity against various insect pests [174]. The insecticidal activity of chitosan and its derivatives was demonstrated against the lepidopterans Spodoptera littoralis [78,175], Helicoverpa armigera, and P. xylostella, and the aphids Aphis gossypii, Metopolophium dirhodum, H. pruni, Rhopalosiphum padi, Sitobium avenae, and M. persicae [176,177]. The mortality of six types of aphids generally ranged between 60% and 80%, with a peak of 99.7%. Furthermore, recent studies showed that a new chitosan derivative, named avermectin-grafted-N,O-carboxymethyl chitosan (NOCC), showed an excellent insecticidal and acaricidal activity against Aphis fabae, Nilaparvata lugens, and Tetranychus cinnabarinus [178].
This entry is adapted from the peer-reviewed paper 10.3390/plants12173152
This entry is offline, you can click here to edit this entry!
