Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
Aortic valve stenosis and malignancy frequently coexist and share the same risk factors as atherosclerotic disease. Data reporting the prognosis of patients with severe aortic stenosis and cancer are limited. Tailoring the correct and optimal care for cancer patients with severe aortic stenosis is complex.
- aortic stenosis
- cancer
- valve replacement
- cardio-oncology
1. Introduction
The coexistence of cancer and calcific aortic valve stenosis (AS) is a common medical scenario, especially in the elderly, due to sharing risk factors (i.e., hypertension, obesity, diabetes, smoking, dyslipidemia), the inflammatory state associated with malignancies, and/or cardiotoxic effects of cancer therapy [1]. As reported in studies listed in Table 1, the prevalence of cancer in patients with severe AS varies between 5.4 and 37.8% [2,3,4]. Data reporting the prognosis of patients with severe AS and cancer are limited. In a 10-year single-center retrospective study, cancer patients with severe AS (mean aortic valve area 1.0 ± 0.3 cm2) had a 5 year mortality of 48%; 59% deaths were due to cancer progression, and 31% were due to heart failure (HF) and stroke [5]. Minamino-Muta et al., in a Japanese retrospective study of 3815 patients in a multicenter AS registry, found that outcomes are worse not only in patients with active cancer but also in those with a previous history of malignancy [6]. Mortality was mainly cancer related, with comparable aortic valve-related deaths between cancer and noncancer patients. Despite the increasing prevalence of AS and cancer, death rates have been steadily declining with the introduction of novel therapies [7], but, at present, the optimal strategy for the management of severe AS in patients with an active cancer is unclear. Cancer patients are routinely excluded from clinical trials because of poor long-term prognosis. Active malignancy often hinders the decision to proceed with invasive procedures, such as cardiac surgery. Furthermore, cancer patients have additional risks due to prior exposure to potentially cardiotoxic chemotherapy, prior chest radiation, immunocompromised state, and increased risk of both bleeding and thromboembolic disease [8]. In patients with cancer, AS may interfere with optimal antineoplastic management (i.e., high-risk oncological surgery or potentially cardiotoxic chemotherapies). Symptomatic AS is occasionally diagnosed in cancer patients undergoing cardiovascular evaluation; likewise, cancer is often recognized during assessments preceding aortic valve interventions. In these complex cases, physicians face a difficult treatment decision.
Table 1. Prevalence of cancer in patients with severe aortic stenosis according to available studies.
| Author | Reference | All Population, n | Cancer, n (%) | Most Frequent Type of Tumor n, (%) |
|---|---|---|---|---|
| Faggiano et al. | [2] | 240 | 64 (26.6%) | na |
| Mangner et al. | [3] | 1821 | 99 (5.4%) | Prostate, 25 (25%) |
| Minamino-Muta et al. | [6] | 3815 | 513 (13.4%) | na |
| Okura et al. | [5] | 26,325 | 111 (0.42%) | Stomach, 13 (14.1%) |
| Guha et al. | [4] | 47,295 | 27,960 (37.8%) | na |
Usually, an echocardiographic evaluation is done before chemotherapy is started; the presence of LV dysfunction before generally represents a risk situation for chemotherapy; on the other hand, LV dysfunction in the presence of severe AS, especially if symptomatic, represents an indication for the treatment of valvular disease soon.
2. Pathophysiology
Clinical risk factors associated with AS development and progression mirror those associated with atherosclerosis, and because many are shared by cancer (advanced age, smoking, hypertension, hypercholesterolemia, obesity, metabolic syndrome, diabetes, and elevated lipoprotein (a) levels) prevalence and incidence rates of both disorders are rising simultaneously [19,20]. These common conditions, together with microbial and viral infections, allergen exposure, radiation, toxic chemicals, alcohol consumption, tobacco use, and other chronic and autoimmune diseases, induce inflammation [21]. It is now known that inflammation mediates all atherosclerosis stages, from initiation to progression and, ultimately, plaque unstabilization and thrombosis. Conditions such as hypertension, smoking, dyslipidemia, and insulin resistance all appear to trigger atherosclerosis, by promoting the expression of adhesion molecules by endothelial cells, allowing leukocyte attachment to blood vessel walls that normally resist their attachment. In recent decades, extensive factual and circumstantial evidence has shown several cancer types to be induced by infection or chronic inflammatory disease (e.g., human papillomavirus and cervical cancer, Helicobacter pylori and stomach cancer, and Epstein–Barr virus and lymphoma) [22]. As stated by Koene et al. [21], controlling cardiovascular disease risk factors can help reduce the risk of cancer. There is an urgent need to improve the health status of the population to reduce the prevalence of both diseases.
Although chronic inflammation is an indispensable feature of the pathogenesis and progression of both cardiovascular disease and cancer, additional mechanisms can be found at their intersection, such as nonmodifiable risk factors, including age, sex, and race/ethnicity, which are uncontrollable. There are obvious differences between male and female organs and hormonal fluctuations that influence both cardiovascular disease and cancer progression. Of all nonmodifiable risk factors, age is a steady independent variable with regard to cardiovascular disease and cancer, yet the associations between age and disease onset can be highly influenced by lifestyle parameters, such as diet, physical activity, body mass index, and smoking.
3. Treatment
Currently, the optimal strategy for the management of severe AS in patients with an active cancer is still unclear. Tailoring the correct and most optimal care for cancer patients with severe AS is complex. Asymptomatic patients with severe AS, in the absence of adverse prognostic features such as reduced LV ejection fraction, or symptoms appearance during an exercise stress test, are recommended for a watchful waiting approach, with regular and frequent follow-up and prompt intervention in case of clinical progression (i.e., symptoms). In this way, asymptomatic patients can proceed with their antineoplastic therapy without interruptions or delays. Medical treatment of hypertension and hyperlipidemia, according to current international guidelines, is recommended for patients with severe AS. According to the 2020 American College of Cardiology Foundation/American Heart Association guidelines, adult patients with symptomatic severe AS (stage D), or with asymptomatic severe AS with LVEF <50%, or need of other cardiac surgery have an indication for aortic valve replacement (AVR). If the risk for a SAVR is high or prohibitive, decision-making focuses on TAVR or palliative care, depending on the life expectancy [23]. In the 2021 European Society of Cardiology Guidelines for The Management of Valvular Heart Disease, AVR is indicated in patients with symptomatic severe AS, except for those in whom the intervention is unlikely to improve quality of life or survival (due to severe comorbidities) or for those with concomitant conditions associated with survival <1 year [24]. In the past years, in patients with severe AS, priority was given to the treatment of neoplastic disease rather than the treatment of severe valvular disease. However, patients undergoing SAVR have shown markedly better survival, due to better resilience to anemia, infection/sepsis, and rapid volume changes from chemotherapy regimens or hypotension/volume loss during surgical procedures, not uncommon during cancer treatment. It must be said, anyway, that patients with severe AS are not excellent candidates for surgery mainly due to comorbidities that increase the estimated periprocedural morbidity and mortality [2]. Conflicting results come from reports where SAVR is performed before cancer surgery [25]. A fundamental problem of SAVR in cancer patients is that open surgery requires extracorporeal circulation. Among various other systemic effects, cardiopulmonary bypass can cause immunosuppression, increase inflammation (as demonstrated by a significant increase in TNF-alpha, Il-10, Il-6, Il-1, and TGF-beta), and worsen cancer outcomes. So, precisely because of the immunosuppressive effects, patients with hematologic cancers are at risk of having worse outcomes than those with solid tumors and better immune systems. However, the relationship between the use of extracorporeal circulation and cancer progression has not yet been clearly demonstrated. Among the comorbidities, we should also consider the vascular fragility that patients with active cancer can develop, and which can sometimes be caused by anticancer drugs or radiotherapy. In addition, cardiac surgery recovery times are longer, and this could lead to a delay and lengthening of the antineoplastic therapy times.
Even if is mandatory to consider each case individually (SAVR vs. TAVR), it is reasonable to conclude that TAVR, for cancer patients with severe AS, can more frequently be the best clinical choice by avoiding cardiopulmonary bypass and all its consequences. One of the biggest advantages of TAVR is its minimal invasiveness and, therefore, shorter recovery time. Moreover, thanks to the increasing access of cancer patients to TAVR, delays in cancer treatment have been significantly reduced from about 2 months after cardiac surgery to 2 weeks [26]. TAVR does not require a median sternotomy or cardiopulmonary bypass and can be performed under local anesthesia, which reduces the overall time required to complete the procedure, which benefits patients with malignancies. Manger et al. and Landes et al. reported that TAVR periprocedural mortality and major complication rates were equivalent in patients with and without active cancer [3]. Kojima et al. reported no difference in terms of complications between patients undergoing TAVR with and without active cancer [27]. However, despite the technical difficulties for open surgery that may be overcome by TAVR, major comorbidities may influence post-TAVR prognosis just as with SAVR [28]. To be eligible for TAVR, as mentioned before, cancer patients should have a prognosis of 1 year or greater. However, precise estimation of prognosis has always been very difficult in these patients, and even more so lately, thanks to the rapid expansion of new and innovative cancer therapies. A fundamental element to consider in choosing the most suitable type of intervention for the patient, in addition to the prognosis, is the stage of neoplastic pathology. Patients with a history of cancer, who are judged in remission by the oncology team, are usually eligible for TAVR. Patients with early cancer stages, who can safely receive oncologic treatment, could be easily considered for TAVR as soon as remission is confirmed. In other cases, performing TAVR before cancer treatment allows for radical oncologic treatment shortly after valve intervention [29]. Patients with AS and a localized cancer can be stabilized and TAVR can be considered after the exclusion of metastatic disease. Patients with advanced disease stage, metastases, multiple comorbidities, and very short estimated survival may be candidates for balloon valvuloplasty as a “bridge to destination” surgery [30].
In the final stages of neoplastic disease, a more conservative approach aimed at improving quality of life during palliative treatment is preferred. A recent expert consensus issued by the Society for Cardiovascular Angiography and Interventions recommends aortic balloon valvuloplasty or TAVR for cancer patients with AS as either a palliative or cure for valvular disease, to improve quality of life or to facilitate appropriate treatment of cancer therapy. Unfortunately, due to the characteristics of advanced stage cancer patients, it is difficult to conduct large studies, limiting the quality of data to support this approach [30]. A small study from Schechter et al. of 65 cancer patients with severe AS found that valve replacement improves survival, regardless of the type of cancer or anticancer therapy, with TAVR being the most effective [26]. Nowadays, the majority of cancer patients diagnosed with severe AS undergo valve replacement before cancer treatment, with the large majority receiving TAVR more than SAVR. Despite the lower risk of TAVR complications, the literature is not univocal about what are the peri-procedural complications of TAVR that could cause a delay in cancer treatment and modify overall survival. A meta-analysis by Marmagkiolis et al. demonstrates a favorable post-TAVR short-term mortality and remarkable safety, with improved stroke and acute kidney injury (AKI) rates without increased bleeding and the need for new pacemaker implantation in cancer patients compared to controls [7]. Conversely, a meta-analysis from Bendary et al., reported higher rates of postprocedural pacemakers, without any difference in short-term mortality [31]. In a systematic review of Arocutipa et al., AKI occurred more frequently in patients with active cancer [32]. AKI is a very common complication of TAVR and can rate in up to 50% of procedures. Its origin is multifactorial: in addition to the iodinated contrast, bleeding and anemia, volume depletion, microembolisms, hypotension, or nephrotoxic drugs also contribute. Importantly, tumor type also plays a role in the risk of post-TAVR AKI. Thus, the decision to ultimately pursue TAVR is not an easy choice and involves a multidisciplinary and holistic approach to assessing the appropriateness of intervention. Recently, also in light of the study of Ullah et al. [33], which highlighted different outcomes between SAVR and TAVR based on the tumor location, researchers proposed a detailed specific decision-making algorithm for the management of symptomatic severe AS in cancer patients, both active and in remission [34]. Specifically, in the case of active cancer, once it is ascertained that cancer-related life expectancy is >1 year, that cancer treatment is not feasible before AS treatment, and that cancer treatment can be delayed for at least 2 months, the decision-making process is comparable to cancer in remission. In this case, evaluation for the presence of high-risk features for SAVR and/or clinical conditions favoring TAVI TAVR is suggested. Where such conditions are not present, the choice between TAVR and SAVR rests in the judgment of the heart team, including the consideration that the tumor site can influence the management strategy and the personal patient choice (Figure 1).
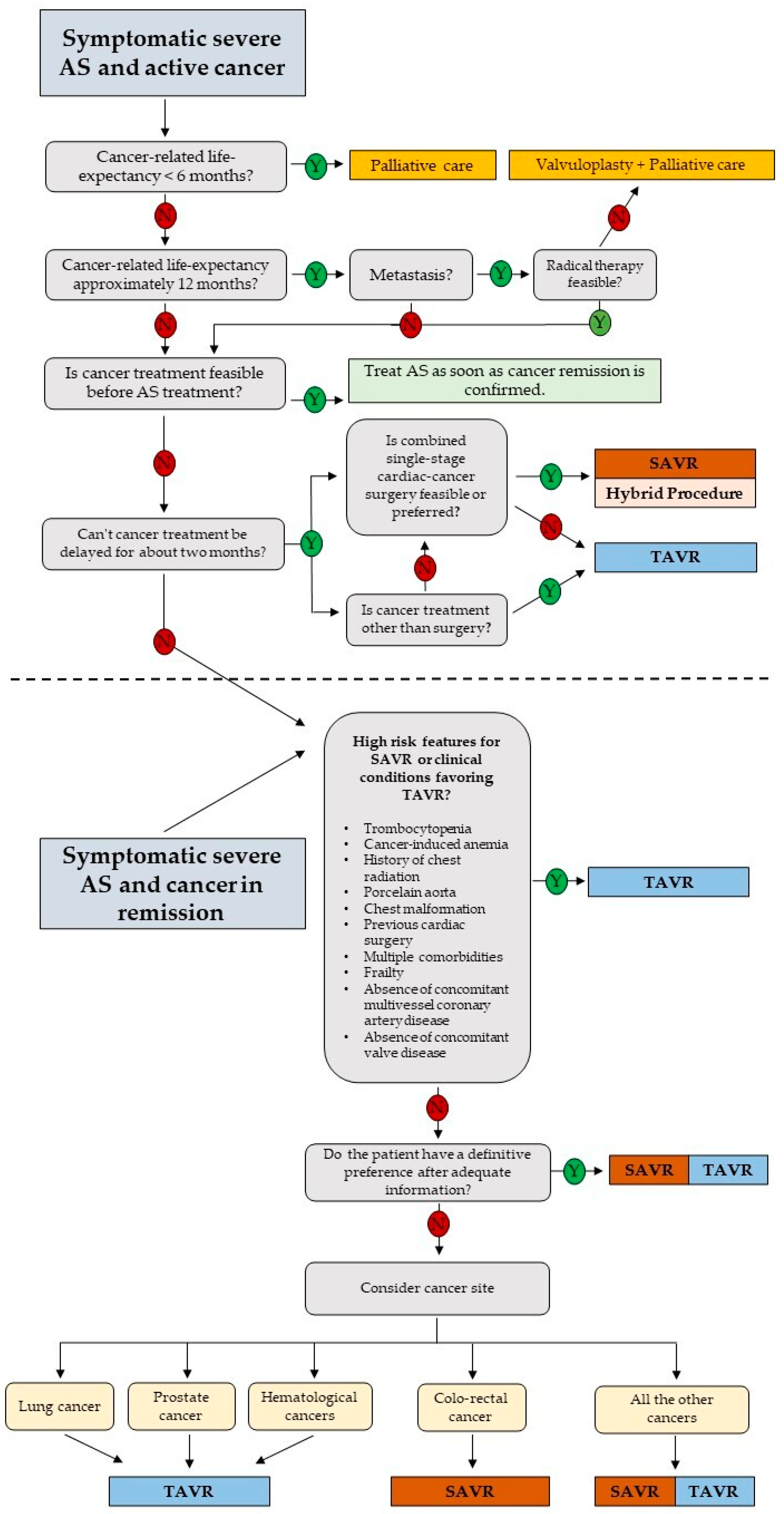
Figure 1. Proposed decision-making algorithm for the management of patients with severe aortic stenosis and cancer. Modified from ref. [34]. AS: aortic valve stenosis; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement.
This entry is adapted from the peer-reviewed paper 10.3390/jcm12185804
This entry is offline, you can click here to edit this entry!
