Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Obstetrics & Gynaecology
Preeclampsia has been termed a “disease of theories” by some as numerous studies have aimed to propose different concepts to explore its complex etiology and pathophysiology.
- preeclampsia
- gestational hypertension
- pregnancy induced hypertension
1. Brief Summary
Preeclampsia has been termed a “disease of theories” by some as numerous studies have aimed to propose different concepts to explore its complex etiology and pathophysiology. Previous findings have suggested that the triggers of preeclampsia include placental factors and other predisposing maternal factors. The mechanisms of early- and late-onset preeclampsia may not be completely the same. Based on the current understanding of preeclampsia, the revised “two-stage model” has become one of the most widely accepted theories regarding its formation.
The classical two-stage model was first described in 1991, innovatively introducing the idea that preeclampsia should be viewed as a trophoblastic disease rather than merely a hypertensive disorder. In this model, the first stage of preeclampsia is described as the “placental stage”, in which deficient remodeling of spiral arteries results in impaired placental perfusion and placental ischemia. As the disease progresses with time and clinical maternal syndrome develops, it reaches the second stage [37]. The clinical manifestations of preeclampsia will be further discussed in the next section.
Ever since the initial proposal of the two-stage theory, ongoing research has expanded and refined the knowledge of the development of preeclampsia. Stage 1 is focused on the revised idea of impaired uteroplacental perfusion with or without poor placentation and subsequent spiral artery insufficiency. Stage 2 surrounds the concept that general endothelial dysfunction and vascular inflammation would lead to a systemic clinical response. Figure 1 displays the contributing factors and the two-stage models of preeclampsia.
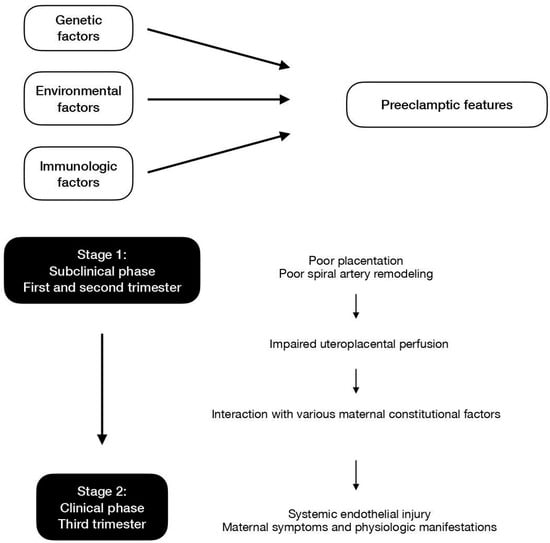
Figure 1. Contributing factors and the two-stage models of preeclampsia.
2. Stage 1
In the current revised model, stage 1 is initiated when reduced placental perfusion develops. Poor placentation and the resultant deficient spiral artery remodeling in the intervillous space is one of the causes for this phase but may not be the sole mechanism. In addition to the impairment of placental perfusion, maternal factors are essential so as to result in the development of systemic maternal pathophysiological changes [37]. Stage 1 usually occurs in the first trimester, at the period of time when the deep invasion of the extravillous trophoblast (EVT) takes place. The migration of EVT cells into the decidua leads to the remodeling of maternal spiral arteries, which is a key element to uteroplacental perfusion and fetal blood supply. The process may initiate as early as before GA 8 weeks, while the establishment of uteroplacental circulation is completed at approximately GA 12 weeks. Hence, it is believed that stage 1 takes place before GA 12 to 20 weeks [38].
The differentiation and invasion of EVTs are regulated by various factors including cytokines, growth factors, chemokines, cell adhesion molecules, placental oxygen tension, extracellular matrix (ECM)-degrading enzymes, and membrane-bound cell surface peptidases. These factors are either directly or indirectly related to the differentiation and decidual invasion of EVT cells and may serve as markers for the first stage of preeclampsia formation [38]. When defective trophoblast invasion and insufficient transformation of the maternal uterine vasculature emerge, decreased maternal uterine blood flow follows, which may be detected and quantified by uterine artery Doppler studies. Persistent high vessel resistance in early pregnancy may suggest that the aforementioned phenomenon has occurred. Existing studies have demonstrated that the placental endothelial cells in women with high-resistance uterine arteries are more sensitive to TNFα and thus are more susceptible to cell injury and apoptosis.
In the normal process of trophoblast invasion, the resistance of uterine artery blood flow decreases, and the uterine artery blood flow increases in the term. The placenta is typically developed in the first trimester. If a relatively hypoxic environment is noted, the latter placental tissues may exhibit an altered balance of antioxidant enzyme activity. However, the histopathology findings of the placenta are nonspecific and not limited to preeclamptic pregnancies. These changes to the placenta can also be induced by other microscopic insults or toxins [39].
3. Stage 2
Stage 2 features the scenario where impaired uteroplacental perfusion interacts with other various maternal constitutional factors. Pathophysiological changes in the liver, kidney, and cardiovascular system are compatible with the concept of insufficient blood supply. Systemic endothelial dysfunction and injury are possible explanations for the maternal clinical manifestations and have been proven to be present in preeclamptic women.
One important issue that attracts interest is how the first stage links to or leads to the second stage. The clinical value of discovering the answer lies in the fact that it may shed light on a way to “prevent” the formation of preeclampsia, which will be further discussed. One proposal suggests that microparticle particles produced during syncytiotrophoblast apoptosis may directly or indirectly result in endothelial dysfunction. An increased amount and concentration of inflammatory cells and substances have been found in women with preeclampsia, and they could potentially alter the systemic endothelial function. The renin-angiotensin system may also play a role in the process. In addition, some recent findings have suggested that vascular endothelial growth factor (VEGF) and placental growth factor (sFlt-1) could be involved in the linkage as well. Moreover, oxidative stress accumulated during the process may provide another possible explanation [40]. Table 1 lists the possible pathways and explanations of mechanisms underlying preeclampsia.
Table 1. Possible pathways and explanations of mechanisms underlying preeclampsia.
| Possible Pathways | Explanations |
|---|---|
| Failure of maternal vascular adaptation |
|
| Dysregulation of the renin-angiotensin system (RAS) |
|
| Oxidative stress |
|
Various factors contribute to the regulation of artery compliance during pregnancy. A failure of maternal vascular adaptation can cause hypertensive disorders such as preeclampsia. Some circulating cytokines and growth factors at abnormal levels may inhibit normal calcium signaling events, thereby damaging cell-to-cell contacts of the endothelium and leading to endothelial dysfunction. Important markers include endothelin-1 (ET-1), interleukin-8 (IL-8), ELAM, and the endothelial leukocyte adhesion molecule-1 [41]. There is also sound evidence of decreased production or bioavailability of nitric oxide (NO)—a stimulant of smooth muscle relaxation—in preeclamptic pregnancies [42]. Other potential influential vasodilators include prostacyclin (PGI2) and the endothelium-derived hyperpolarizing factor (EDHF) [41].
As mentioned above, the dysregulation of the renin-angiotensin system (RAS) may participate in the pathogenesis of preeclampsia. In 2007, Florian Herse et al. published a study that included preeclamptic and non-preeclamptic women who had undergone cesarean deliveries. Genetic characteristics and histopathological results of the maternal and placental tissues of the participants were investigated. A 4-fold increase in the angiotensin II type 1 (AT1) receptor in the decidua was found in preeclamptic pregnancies. Increases in corresponding gene and protein expression were also confirmed by RT-PCR and immunohistochemistry studies. Circulating agonistic autoantibodies (AAs) targeting the AT1 receptor have been described previously, with the ability to cross the placenta and enter fetal circulation. AT1-AAs could induce calcium signaling and initiate events that would later lead to preeclampsia [43]. Roxanna A. Irani et al. published a study with similar findings in 2010. Animal experiments showed that pregnant mice with AT1-AA injections developed preeclamptic features and also had increased levels of antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt-1) and endoglin. Additionally, AT1-AA might be associated with increased TNF-α, indirectly causing damage to the endothelium and end organs [44].
Oxidative stress describes the imbalance between the formation of oxidative reactive species (ROS) and the antioxidant capacity of the body [45]. A causal role of oxidative stress in hypertension has been demonstrated in previous research, with multiple possible pathogenic pathways including the alteration of NO bioavailability or signaling. A reduction of oxidative stress has been observed in hypertensive cases who received antihypertensive treatment [46]. Oxidative stress in the healthy placenta may be important for its organogenesis, but excess levels in the impaired placenta would lead to increased circulating placenta debris, damaging the maternal endothelial cells in the term. As a major source of ROS production, the mitochondria have been found to be swollen in the trophoblasts of preeclamptic animal models, which plays a crucial role in cell apoptosis. Altogether, any errors in the maintenance of the oxygen pressure may bring about placental diseases and maternal complications, such as preeclampsia [46].
4. Limitations of the Placenta Model
Even though the two-stage theory is the mainstream explanation of the origin of preeclampsia, some argue that further evaluation is needed to determine the causative relation between trophoblast development and spiral artery transformation. For instance, previous case reports have pointed out similar findings of the uterine artery Doppler waveforms in extra-uterine pregnancies, suggesting that the resistance of uterine artery blood flow may not accurately reflect the consequences of trophoblast invasion [47].
Some have suggested that the result of Doppler studies may be a reflection of systemic vascular resistance changes but not on the uterine artery itself. The argument is based on the paradox that a “de-transformation” of spiral arteries does not occur when the vascular resistance of the uterine artery is noted in the third trimester [48]. In the meantime, while it is fairly certain that impaired uteroplacental perfusion is associated with subsequent endothelial dysfunction, almost all the supporting evidence of different hypotheses of its linkage raises some challenges. To date, it is believed that many potential mechanisms underlie preeclampsia, and the disease is caused by complicated interactions between maternal and environmental factors, and potentially more than that. The incomplete understanding of its pathogenesis continues to provoke further research.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph20042994
This entry is offline, you can click here to edit this entry!
