Corneal clarity is required for vision, and blindness occurs when the cornea becomes opaque. The cornea is covered by unique transparent epithelial cells that serve as an outermost cellular barrier bordering between the cornea and the external environment. Corneal sensory nerves protect the cornea from injury by triggering tearing and blink reflexes, and are also thought to regulate corneal epithelial renewal via unknown mechanism(s). When protective corneal sensory innervation is absent due to infection, trauma, intracranial tumors, surgery, or congenital causes, permanent blindness results from repetitive epithelial microtraumas and failure to heal. The condition is termed neurotrophic keratopathy (NK), with an incidence of 5:10,000 people worldwide.
1. Introduction
Corneal clarity is required for vision, and blindness occurs when it becomes opaque. Similar to the epidermis of the skin, the superficial corneal epithelium is continually sloughed off and replaced as it shields the eye from external insults [
1]. Corneal epithelial renewal [
2,
3] depends on the activity of limbal stem cells (LSCs) [
4,
5,
6,
7] that are located in the basal epithelium of the limbus, surrounding the cornea [
6,
8]. The limbus, which contains the Palisades of Vogt, forms the transition zone between the cornea and conjunctiva [
1,
9]. In the limbal niche, the LSCs interact with mesenchymal stromal cells (MSCs; are also addressed as limbal niche cells) and T cells that play a critical role in the maintenance and activity of the LSCs [
10,
11,
12]. Normally, the LSCs reside in a growth-arrested or slow-cycling state [
13,
14,
15,
16], exhibit morphological characteristics of stem cells, and express genes associated with asymmetric cell division [
17,
18,
19,
20,
21]. During homeostatic epithelial renewal or after injury, the LSC progeny differentiate to transient amplifying cells (TACs) that migrate radially to the central cornea where they differentiate further into epithelial cells, thereby replenishing the corneal epithelium [
11,
12,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32] (
Figure 1).
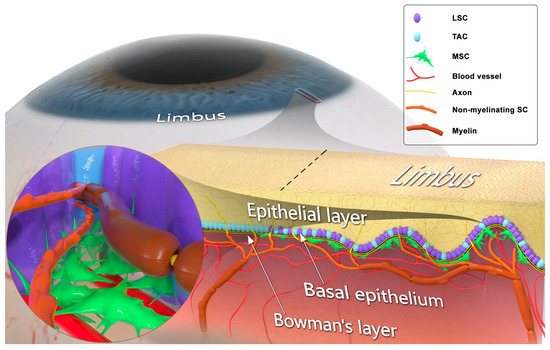
Figure 1. Structural and cellular composition of the limbal niche. The corneoscleral limbus contains limbal epithelial crypts, forming the limbal niche (or limbus). Limbal stem cells (LSCs) differentiate into transient amplifying cells (TACs) and, together with TACs, form the basal epithelial cellular layer. LSCs lie in close contact with mesenchymal stromal cells (MSCs), myelinating Schwann cells (SCs), ensheathing Aδ fibers, and non-myelinating SCs associated with C fibers. LSC, MSC, and SC populations engage in juxtaparacrine molecular crosstalk. The limbal MSCs are attached to the basement membrane, interact with blood vessels, and their projections pass through the basement membrane in contact with LSCs. SCs associated with unmyelinated sensory axons penetrate the basement membrane and extend neurites, which terminate in the epithelial surface.
The cornea is innervated by sensory nerves that protect it from injury by activating tearing and blink reflexes [
33]. The axons in the basal epithelial layer of the limbus run adjacent to the LSCs, and their free nerve endings contact epithelial cells [
34]. There is a growing volume of evidence suggesting a critical role of corneal sensory innervation in regulating its epithelial renewal via stimulating the activity of LSCs [
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51]. When protective sensory innervation is lost after infection, trauma, intracranial tumors or ocular surgery, or when innervation fails to develop in congenital cases, permanent blindness results from repetitive microtraumas that induce epithelial breakdown, ulceration, scarring, and opacification of the cornea [
36,
38,
52,
53] (
Figure 2). This condition, known as neurotrophic keratopathy (NK), affects nearly 5/10,000 people [
54,
55]. Patients with NK develop persistent breakdown of the epithelium [
36,
38,
52,
53] and poor healing [
53,
56,
57,
58,
59] that, in turn, inevitably cause scarring and opacification of the cornea [
54,
60,
61,
62,
63]. The field’s prevailing conceptual model is that corneal epithelial wound healing and maintenance depend on sensory axons providing direct trophic stimulation of LSCs [
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51].
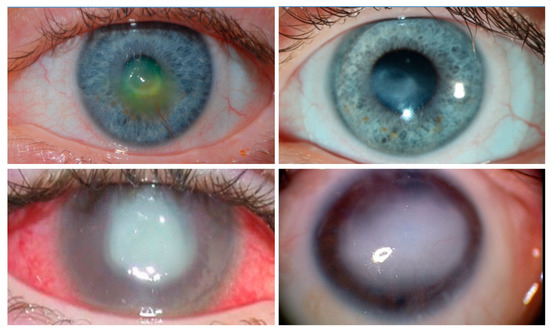
Figure 2. Clinical images of neurotrophic keratopathy (NK). Clinical appearance of anesthetic corneas, showing central corneal opacity with overlying epitheliopathy. Conjunctival injection, corneal neovascularization, and corneal stromal scar with overlying epithelial defect are demonstrated. (
Top left): A cornea with a shallow corneal ulcer. The clinician has applied fluorescein, which indicates the location of the de-epithelialized corneal stroma. (
Top right): Same patient as (
Top left). The ulcerated area has progressed onwards to scarring as the epithelial defect heals. (
Bottom left): A patient with severe NK leading to corneal perforation and infection. (
Bottom right): A patient with recurrent ulcerations leading to total opacification of the cornea. All four of these patients were treated with corneal neurotization.
2. Corneal Sensory Innervation
The sensory innervation of the cornea protects against environmental microtraumas by triggering tearing and blink reflexes [
33]. Given the crucial role of vision in survival and the necessity of corneal transparency for vision, the cornea is the most highly sensory nerve-innervated region of the body’s surface [
72]. Trigeminal nerve fibers enter the cornea via the suprachoroid space and extend in a radial pattern parallel to the corneal surface. Stemming from their annular plexus at the periphery, these nerves terminate as free nerve endings in the limbal region, forming the limbal plexus [
34]. The axons in the basal epithelial layer of the limbus run adjacent to the LSCs, with their free nerve endings making contact with epithelial cells [
34] (
Figure 1). Nerve fibers from the deeper stroma proceed towards the epithelium to form the subepithelial nerve plexus, and then ascend to Bowman’s layer and the basement membrane, dividing into thinner fibers that form the sub-basal nerve plexus. Finally, fibers from the latter plexus penetrate the epithelial layer (
Figure 1) forming whorl-like or vortex patterns on the corneal epithelium’s surface [
34,
73] (
Figure 3).
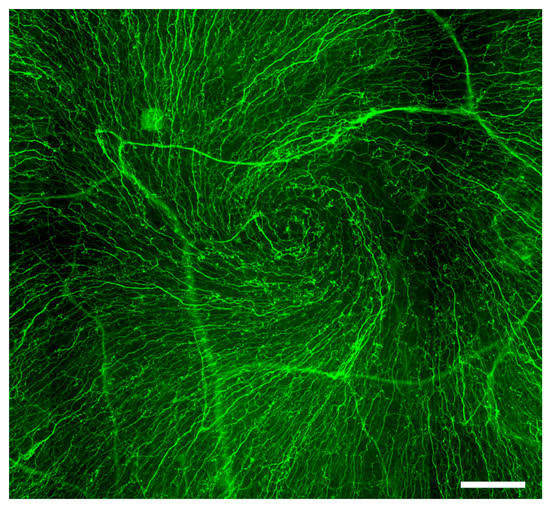
Figure 3. Whorl-like or vortex pattern of the corneal epithelial nerves. Immunofluorescent image of βIII-tubulin-stained whole-mount rat cornea demonstrates neurites of the corneal sensory axons forming a whorl-like or vortex pattern on the corneal epithelium’s surface. Scale bar: 100 μm.
Most epithelial nerve fibers are polymodal non-myelinated C fibers that convey sharp pain in response to microtraumas and chemical stimulation. The remaining nerve endings are myelinated Aδ fibers that are responsive to cold stimuli [
34,
72]. These nerve fibers retain their myelination at the limbus and the peripheral part of the stroma, but gradually lose the myelin sheath as they approach the center of the cornea [
34].
3. Schwann Cells and Innervation-Dependent Corneal Epithelial Renewal
Multiple studies addressed the importance of the limbal niche in regulating LSC maintenance and activity. MSCs and T cells are the key cellular components of the niche, regulating self-renewal, proliferation, and dedifferentiation of LSCs [
10,
11,
12]. Following limbal injury, the niche is also capable of inducing the dedifferentiation of terminally committed epithelial cells to replenish the pool of LSCs [
23]. Paradoxically, despite the well-known critical role of corneal innervation, no attempts had been made until recently to find the linking factor(s) that connect between the corneal nerves and the activity of the limbal niche.
One previously prevalent school of thought argued that corneal epithelial stem cells differentiate vertically to replenish the upper layers of the epithelial cells [
77]. More recent reports, using single-cell RNA sequencing (scRNA-seq) and lineage tracing approaches in mice, unequivocally showed that LSCs are restricted to the limbal niche, which is also the mostly richly innervated part of the cornea [
11,
12,
22,
23,
24] (
Figure 1).
In the limbus, axons of the cornea-innervating nerves are associated with myelinating and non-myelinating SCs in a 1:1 relation, and are situated in close vicinity to LSCs (
Figure 1) [
71]. In the peripheral nerve system, in addition to the well-studied role of SCs in axonal trophic support and electrophysiological activity, SCs in nerve terminals play a key role in regulating tissue regeneration and homeostatic stem cells activity [
69,
70,
78]. In mammalian wounded skin, mature SCs dedifferentiate into SC precursors that produce cytokines and growth factors, which mediate wound closure [
70].
The comprehensive analysis performed by Mirmoeini et al. provides important insight into the regulatory complexity of innervation-dependent corneal epithelial renewal. The breakthrough findings provide the missing linking factor by suggesting SCs as a new cellular component of the limbal niche that mediates corneal innervation-dependent epithelial renewal [
71].
4. Trophic Regulation of Sensory Innervation-Mediated Corneal Epithelial Renewal
Deficiencies in the signaling molecules, including neurotrophins and neurotransmitters, is a possible, if not a likely contributor to the demise of the epithelium after corneal denervation, because the deficiency results in a loss of regenerative capacity and progressive epithelial degeneration [
36,
37,
75,
76]. Concentrations of acetylcholine and the neuropeptide substance P in the corneal epithelium and tears have been shown to decline after corneal denervation [
36,
82,
83]. In several in vitro experiments, pro-proliferative and cell migration-promoting effects of neuropeptides or neurotrophins, such as substance P in combination with IGF-1, have been observed in corneal epithelial cells and limbal stem cells [
84,
85,
86].
New insight to the mechanistic understanding of the regulation of LSCs during corneal epithelial renewal arrived from a recently reported comparative scRNA-seq analysis of dissociated corneal limbi that were harvested from healthy, de-epithelialized healing and denervated corneas [
71]. A complex regulatory trophic communication between limbal cell populations was proposed on the basis of the altered gene expression in the pathological conditions [
71]. This model of paracrine interactions suggests that NGF acts synergistically with other trophic factors, including CNTF, PDGF-α, and TGF-β, locally expressed by SCs and MSCs in the limbus, to regulate the activity of LSCs.
5. Currently Available Pharmacological Approaches to Treat NK
The current nonsurgical (i.e., conservative) treatments for NK include autologous serum topical applications [
93], topical nerve growth factor (see below), as well as lubricating agents, such as artificial tears. Less expensive growth factors, such as topical insulin (1 U/mL applied three times per day) have also been used to accelerate healing of epithelial defects in patients with NK [
94,
95]. Others have used scleral lenses to slow the progression of NK. Surgical options have historically relied on tarsorrhaphy (suturing the eyelids together) to reduce the area of corneal surface exposed, and hence reducing evaporative losses and protecting the ocular surface. These methods help reduce the incidence of corneal ulceration in NK; however, many patients will eventually sustain a corneal ulcer despite these treatments.
Nerve Growth Factor (NGF)
Thus far, recombinant human (rhNGF; Cenegermin, Dompé, Milan, Italy) remains the only clinically approved topical treatment for NK [
66,
97]. However, despite its low toxicity and evidence for NGF’s stimulation of LSC activity [
98] and corneal epithelial healing in human NK cases [
50,
99,
100], NGF treatment of NK presents remaining concerns. Firstly, the treatment requires extremely high doses, prolonged treatment duration, and dosing every two hours. Secondly, NGF treatment fails in over 30% of patients, especially in those with more severe NK [
66,
97]. These observations suggest that NGF alone may not be sufficient to fully compensate for a lack of innervation in NK patients.
In preclinical in vivo studies, the topical application of NGF significantly accelerated the healing of epithelial defects in physiologically innervated corneas, and conversely, topical application of NGF antibodies reduced the rate of healing [
49,
102]. Placebo-controlled clinical studies that evaluated the therapeutic effect of topically applied NGF in patients with the corneal lesions of NK found significantly faster epithelial regeneration compared to the placebo group. There was a concomitant significant increase in corneal sensitivity, which suggested that the cornea had become reinnervated and that the reinnervation may be induced by the applied NGF [
48,
103].
NGF significantly increases the expression, axonal transport, and secretion of certain neuropeptides such as substance P in sensory neurons, suggesting a possible pathophysiological connection between the observed effects of the trophic factor and neurotransmitters [
104]. The detection of NGF and its corresponding high-affinity receptor tropomyosin kinase A (TrkA) in corneal epithelial cells and LSCs in humans and murine species suggests partial autocrine or paracrine effects of NGF in the epithelium and LSCs [
48,
49].
The expression of TrkA by both corneal sensory neurons and non-neuronal cells, and the local expression of other trophic factors rather than NGF suggested to be involved in corneal epithelial renewal, raise two possible explanations for the observed inconsistencies in the efficacy of NGF treatment in NK.
6. Strategies to Promote Corneal Reinnervation
6.1. Potential Treatment with Tacrolimus
Tacrolimus, a clinically approved immunosuppressant commonly used for various ophthalmic conditions [
121], including allergic keratoconjunctivitis [
122] and corneal transplantation [
123], has been shown to promote axon regrowth in vitro [
124,
125,
126,
127] and axonal regeneration following nerve injury in vivo [
128,
129,
130,
131,
132]. The direct neurotrophic effect of tacrolimus is mediated by the chaperone-like FK506 binding protein (FKBP52) [
125,
127,
133,
134], which forms heterocomplexes with the 90 kDa heat-shock protein (Hsp90) and its co-chaperone p23 [
134] in the neuronal nucleus. Injured neurons redistribute this complex to the growth cones of regenerating neurites upon cellular contact with tacrolimus, promoting their accelerated regeneration [
134]. FKBP52 also mediates neuronal growth cone guidance in response to attractive and repulsive chemotactic signals [
135]. The systemic delivery of tacrolimus accelerates axonal regeneration in vivo by 12% to 16% [
136,
137]. Sustained local delivery of low-dose tacrolimus directly at the repair site of peripheral nerves increases the number of regenerating nerve fibers and accelerates their rate of regeneration [
138,
139,
140].
Given the well-documented clinical safety record of tacrolimus in ophthalmic applications [
141,
142,
143] as well as in other indications [
144,
145,
146,
147,
148], this therapeutic approach could be seamlessly implemented in clinical trials. Furthermore, although a specialized drug delivery system designed for the sustained topical delivery of tacrolimus may not be necessary for preliminary clinical investigations, tacrolimus eye drops may be considered for self-administration during the day, albeit with a mean surface residence time of over 1.5 h [
149]. It remains to be seen whether this frequency of dosing is adequate for maintaining therapeutic levels of tacrolimus in the cornea, and future investigations will shed light on this topic.
6.2. Corneal Neurotization
Clinically, corneal “neurotization” surgery improves corneal wound healing and corneal innervation in patients with anesthetic corneas [
64]. Neurotization entails harvest and placement of healthy functional nerves into a previously denervated tissue to regain motor or sensory function. This approach was first reported in the peer-reviewed literature by Terzis et al., who used direct neurotization from the contralateral supratrochlear (STN) and supraorbital nerves (SON) [
150]. These nerves were transferred directly into the denervated cornea’s limbal area, which provided corneal sensation and enabled corneal healing in patients with anesthetic corneas. Later modifications of this procedure, which utilized nerve grafts to reduce invasiveness and allow for more distant sensory nerve sources, were similarly successful and made the procedure feasible for bilaterally affected patients and congenital patients with more widespread facial denervation [
151] (
Figure 4).
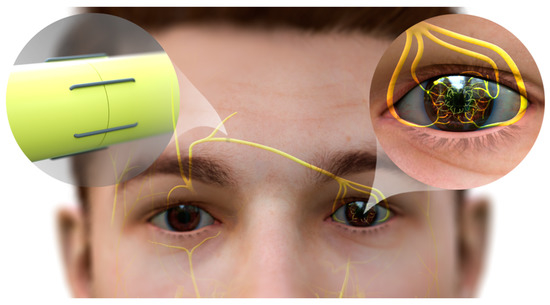
Figure 4. Neurotization of human cornea. The end of the sural nerve graft is coapted to the divided donor supraorbital nerve, permitting fibers to regenerate into the nerve graft. The nerve graft is passed subcutaneously from this incision into the contralateral upper lid counterincision. The graft is then passed into a subconjunctival plane and separated into its component fascicles, which are then tunneled and secured individually around the limbus. Axons of the donor nerve emerge from the graft, and grow into the corneal stroma and into the overlying epithelium. While most corneal neurotization patients gain sensation post-neurotization, many do not achieve the highest possible level of sensation as measured by standard Cochet–Bonnet esthesiometry (CBA, a variable length nylon monofilament). Those patients that achieved less than 50 mm of CBA (of maximum 60 mm) were not as protected against recurrent corneal ulceration post-operatively as those who achieved 60 mm on CBA [
152]. This observation suggests that corneal neurotization, while revolutionary, may not be sufficient to provide full corneal sensation in many cases, leaving room for assistance from topical therapeutics.
This entry is adapted from the peer-reviewed paper 10.3390/ijms241612615